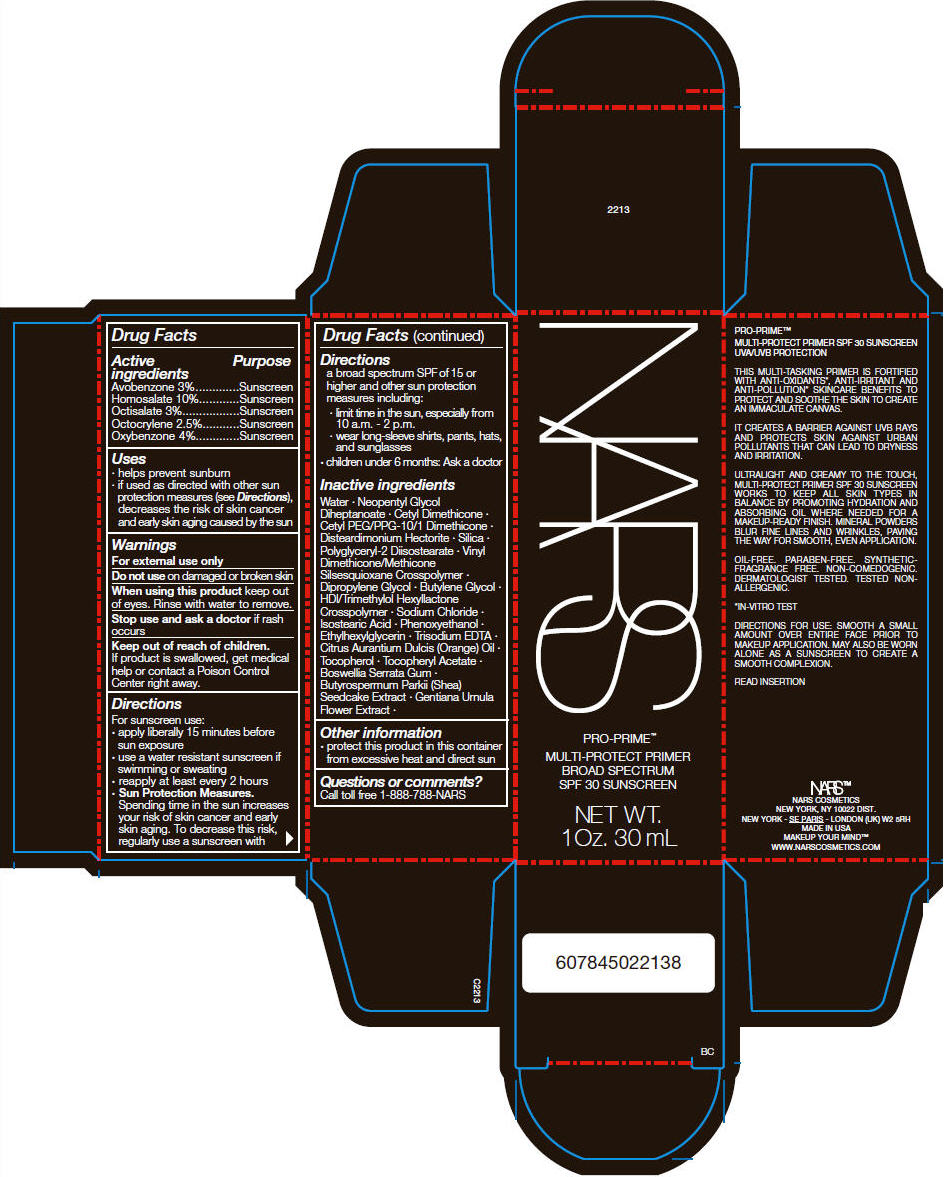
NARS PRO-PRIME MULTI-PROTECT PRIMER- avobenzone, homosalate, octisalate, octocrylene, and oxybenzone cream
NARS PRO-PRIME MULTI-PROTECT PRIMER by
Drug Labeling and Warnings
NARS PRO-PRIME MULTI-PROTECT PRIMER by is a Otc medication manufactured, distributed, or labeled by NARS Cosmetics, SHISEIDO AMERICA INC., Davlyn Industries Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
Water Neopentyl Glycol Diheptanoate Cetyl Dimethicone Cetyl PEG/PPG-10/1 Dimethicone Disteardimonium Hectorite Silica Polyglyceryl-2 Diisostearate Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer Dipropylene Glycol Butylene Glycol HDI/Trimethylol Hexyllactone Crosspolymer Sodium Chloride Isostearic Acid Phenoxyethanol Ethylhexylglycerin Trisodium EDTA Citrus Aurantium Dulcis (Orange) Oil Tocopherol Tocopheryl Acetate Boswellia Serrata Gum Butyrospermum Parkii (Shea) Seedcake Extract Gentiana Urnula Flower Extract
- Other information
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
NARS PRO-PRIME MULTI-PROTECT PRIMER
avobenzone, homosalate, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13734-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 922 mg in 30.72 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 3072 mg in 30.72 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 922 mg in 30.72 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 768 mg in 30.72 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1229 mg in 30.72 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIPROPYLENE GLYCOL (UNII: E107L85C40) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SODIUM CHLORIDE (UNII: 451W47IQ8X) ISOSTEARIC ACID (UNII: X33R8U0062) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) EDETATE TRISODIUM (UNII: 420IP921MB) ORANGE OIL (UNII: AKN3KSD11B) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) GENTIANA URNULA FLOWER (UNII: 2RIZ3HY23X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13734-101-60 1 in 1 CARTON 1 30.72 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 12/01/2012 Labeler - NARS Cosmetics (837363571) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 MANUFACTURE(13734-101) , ANALYSIS(13734-101) Establishment Name Address ID/FEI Business Operations Davlyn Industries Inc 624436254 MANUFACTURE(13734-101)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.