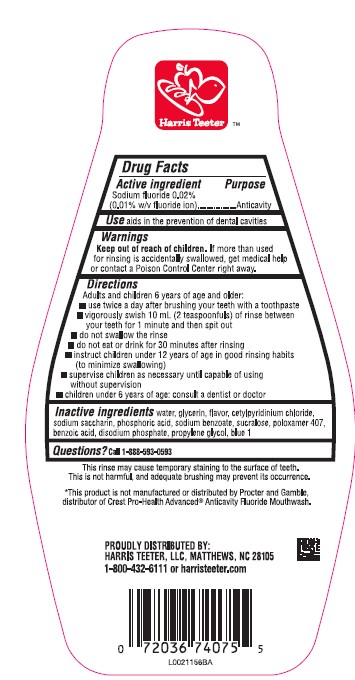
ORAL ANTIVAVITY- sodium fluoride mouthwash
Oral Antivavity by
Drug Labeling and Warnings
Oral Antivavity by is a Otc medication manufactured, distributed, or labeled by HARRIS TEETER, LLC, Vi-Jon, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
- Warnings
- Keep out of reach of children.
-
Directions
Adults and children 6 years of age and older:
- use twice daily after brushing your teeth with a toothpaste
- vigorously swish 10 mL (2 teaspoonfuls) of rinse bwtween your teeth for 1 minute then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- instruct children under 12 years of age in good rinsing habits (to minimize swallowing)
- supervise children as necessary until capable of using witout supervision
- children under 6 years of age: consult a dentist or doctor
- Inactive ingredients
- Questions?
- Claims/Disclaimer
- Adverse Reactions
- Principal display panel
-
INGREDIENTS AND APPEARANCE
ORAL ANTIVAVITY
sodium fluoride mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72036-576 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PHOSPHORIC ACID (UNII: E4GA8884NN) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) POLOXAMER 407 (UNII: TUF2IVW3M2) BENZOIC ACID (UNII: 8SKN0B0MIM) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72036-576-86 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/08/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/08/2015 Labeler - HARRIS TEETER (047279351) Registrant - Nice-Pak Products, LLC (119091520) Establishment Name Address ID/FEI Business Operations Nice-Pak Products, LLC 119091514 manufacture(72036-576)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.