NALOXONE HYDROCHLORIDE spray
NALOXONE HYDROCHLORIDE by
Drug Labeling and Warnings
NALOXONE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Strategic Sourcing Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient (in each spray)
- Purpose
- Uses
-
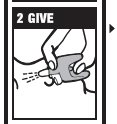
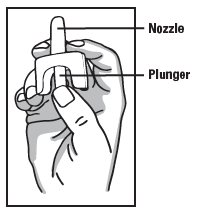
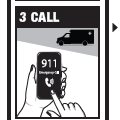
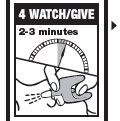
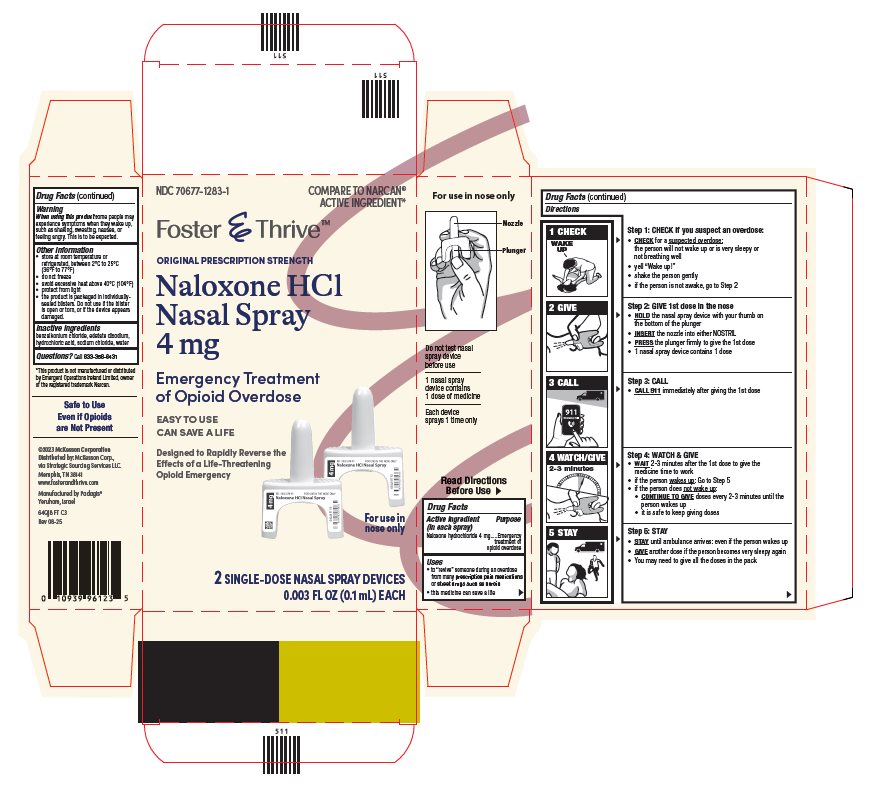
Directions
Emergency Treatment of Opioid Overdose
Important:
▪ For use in the nose only
▪ Do not test nasal spray device before use
▪ 1 nasal spray device contains 1 dose of medicine
▪ Each device sprays 1 time only
For opioid emergencies, call 911. For questions on Naloxone HCl Nasal Spray 4 mg, call Padagis® at 1-866-634-9120 or go to www.padagis.com.
- Warning
- Other information
- Inactive Ingredients
- Questions?
-
Package/Label Principal Display Panel
NDC: 70677-1283-1
COMPARE TO NARCAN® ACTIVE INGREDIENT*
Foster & Thrive™
Original Prescription Strength
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid Overdose
EASY TO USE
CAN SAVE A LIFE
Designed to Rapidly Reverse the Effects of a Life-Threatening Opioid Emergency
For use in nose only
2 SINGLE-DOSE NASAL SPRAY DEVICES
0.003 FL OZ (0.1mL) EACH
*This product is not manufactured or distributed by Emergent Operations Ireland Limited, owner of the registered trademark Narcan.
Safe to Use Even if Opioids are Not Present
©2023 McKesson Corporation
Distributed by: McKesson Corp.,
via Strategic Sourcing Services LLC.
Memphis, TN 38141
www.fosterandthrive.com
Manufactured by Padagis®
Yeruham, Israel
64QJ8 FT C3
Rev 08-25
64Q00 RT QS4
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70677-1283 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70677-1283-1 2 in 1 CARTON 04/01/2025 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211951 04/01/2025 Labeler - Strategic Sourcing Services LLC (116956644)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.