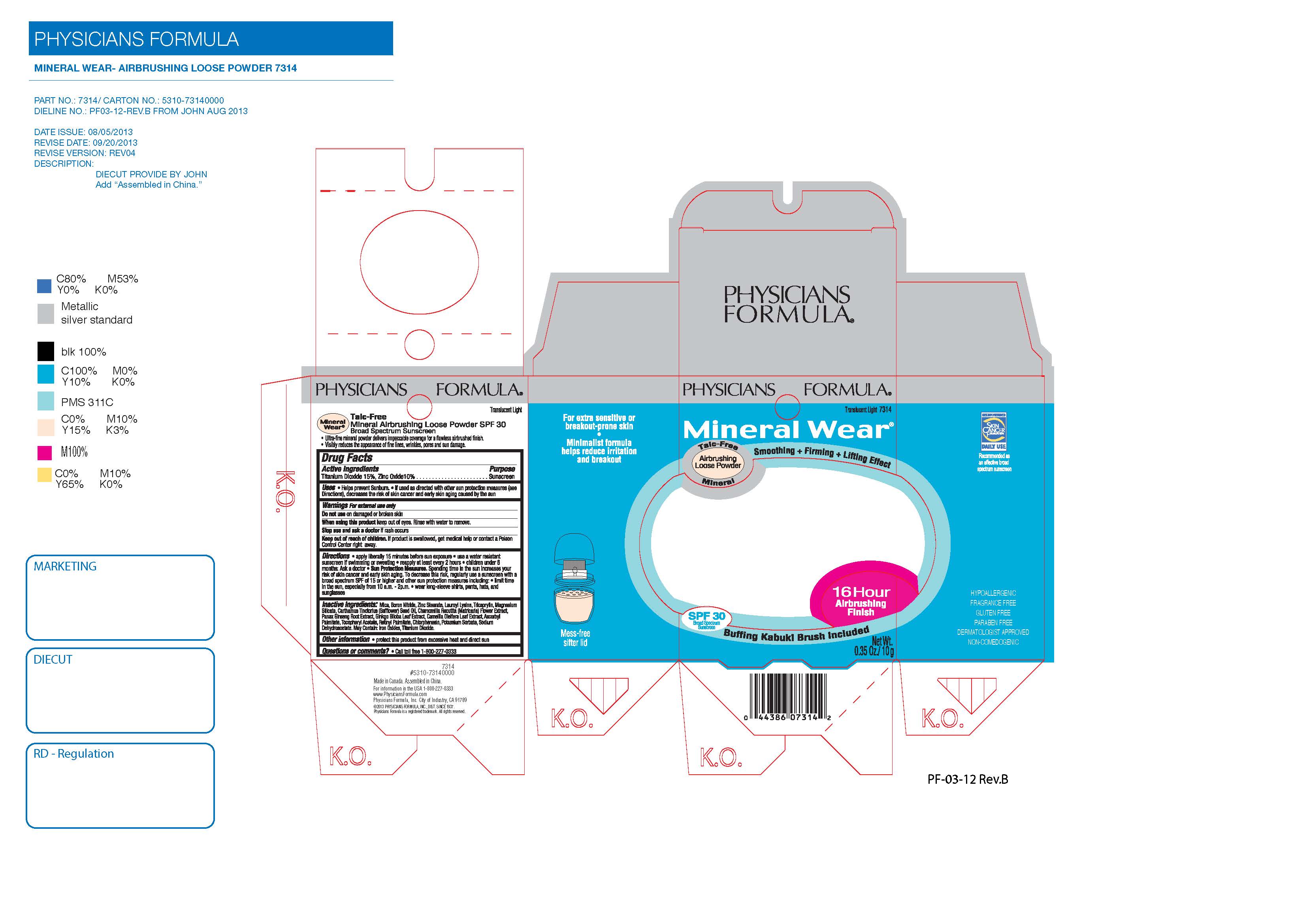
MW Airbrushing Loose Pwdr-PF-CC
Mineral Wear Talc-Free Airbrushing Loose Powder by
Drug Labeling and Warnings
Mineral Wear Talc-Free Airbrushing Loose Powder by is a Otc medication manufactured, distributed, or labeled by Physicians Formula Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MINERAL WEAR TALC-FREE AIRBRUSHING LOOSE POWDER SPF 30- titanium dioxide, zinc oxide powder
Physicians Formula Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
MW Airbrushing Loose Pwdr-PF-CC
| MINERAL WEAR TALC-FREE AIRBRUSHING LOOSE POWDER
SPF 30
titanium dioxide, zinc oxide powder |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Physicians Formula Inc (021261805) |
Revised: 12/2019
Document Id: 9a04875a-801a-b3d4-e053-2995a90ab8a4
Set id: 57a0cc92-1fe5-473e-ad81-43f143626295
Version: 6
Effective Time: 20191218
Physicians Formula Inc