TRODELVY- sacituzumab govitecan powder, for solution
TRODELVY by
Drug Labeling and Warnings
TRODELVY by is a Prescription medication manufactured, distributed, or labeled by Immunomedics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRODELVYTM safely and effectively. See full prescribing information for TRODELVYTM
TRODELVYTM (sacituzumab govitecan-hziy) for injection, for intravenous use
Initial U.S. Approval: 2020WARNING: NEUTROPENIA AND DIARRHEA
- Severe neutropenia may occur. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Consider G-CSF for secondary prophylaxis. Initiate anti-infective treatment in patients with febrile neutropenia without delay. (5.1)
- Severe diarrhea may occur. Monitor patients with diarrhea and give fluid and electrolytes as needed. Administer atropine, if not contraindicated, for early diarrhea of any severity. At the onset of late diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide. If severe diarrhea occurs, withhold TRODELVY until resolved to < Grade 1 and reduce subsequent doses. (2.3, 5.2)
INDICATIONS AND USAGE
TRODELVY is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for metastatic disease. (1)
This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1, 14)
DOSAGE AND ADMINISTRATION
- Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38. (2.1)
- For intravenous infusion only. Do not administer as an intravenous push or bolus.
- The recommended dose is 10 mg/kg once weekly on Days 1 and 8 of continuous 21-day treatment cycles until disease progression or unacceptable toxicity. (2.2)
- Premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting is recommended. (2.2)
- Monitor patients during the infusion and for at least 30 minutes after completion of infusion. Treatment interruption and/or dose reduction may be needed to manage adverse reactions. (2.2)
- See Full Prescribing Information for preparation and administration instructions. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 180 mg lyophilized powder in single-dose vials for reconstitution. (3)
WARNINGS AND PRECAUTIONS
- Hypersensitivity: Hypersensitivity reactions including severe anaphylactic reactions have been observed. Monitor patients for infusion-related reactions. Permanently discontinue TRODELVY if severe or life-threatening reactions occur. (5.3)
- Nausea/Vomiting: Use antiemetic preventive treatment and withhold TRODELVY for patients with Grade 3 nausea or Grade 3-4 vomiting at the time of scheduled treatment. (5.4)
- Patients with Reduced UGT1A1 Activity: Individuals who are homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia following initiation of TRODELVY treatment. (5.5)
- Embryo-Fetal Toxicity: TRODELVY can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥25%) in patients with mTNBC are nausea, neutropenia, diarrhea, fatigue, anemia, vomiting, alopecia, constipation, rash, decreased appetite, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Immunomedics, Inc. at 1-888-983-4668 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NEUTROPENIA AND DIARRHEA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
2.2 Recommended Dose and Schedule
2.3 Dose Modifications for Adverse Reactions
2.4 Preparation for Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Neutropenia
5.2 Diarrhea
5.3 Hypersensitivity
5.4 Nausea and Vomiting
5.5 Use in Patients with Reduced UGT1A1 Activity
5.6 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TRODELVY
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NEUTROPENIA AND DIARRHEA
- Severe neutropenia may occur. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 or neutropenic fever. Monitor blood cell counts periodically during treatment. Consider G-CSF for secondary prophylaxis. Initiate anti-infective treatment in patient with febrile neutropenia without delay [see Warnings and Precautions (5.1)].
- Severe diarrhea may occur. Monitor patients with diarrhea and give fluid and electrolytes as needed. Administer atropine, if not contraindicated, for early diarrhea of any severity. At the onset of late diarrhea, evaluate for infectious causes and, if negative, promptly initiate loperamide [see Warnings and Precautions (5.2)]. If severe diarrhea occurs, withhold TRODELVY until resolved to < Grade 1 and reduce subsequent doses [see Dosage and Administration (2.3)].
-
1 INDICATIONS AND USAGE
TRODELVY is indicated for the treatment of adult patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior therapies for metastatic disease.
This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Use Information
Do NOT substitute TRODELVY for or use with other drugs containing irinotecan or its active metabolite SN-38.
2.2 Recommended Dose and Schedule
The recommended dose of TRODELVY is 10 mg/kg administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles. Continue treatment until disease progression or unacceptable toxicity. Do not administer TRODELVY at doses greater than 10 mg/kg.
Administer TRODELVY as an intravenous infusion only. Do not administer as an intravenous push or bolus.
First infusion: Administer infusion over 3 hours. Observe patients during the infusion and for at least 30 minutes following the initial dose, for signs or symptoms of infusion-related reactions [see Warning and Precautions (5.3)].
Subsequent infusions: Administer infusion over 1 to 2 hours if prior infusions were tolerated. Observe patients during the infusion and for at least 30 minutes after infusion.
Premedication
Prior to each dose of TRODELVY, premedication for prevention of infusion reactions and prevention of chemotherapy-induced nausea and vomiting (CINV) is recommended.
- Premedicate with antipyretics, H1 and H2 blockers prior to infusion, and corticosteroids may be used for patients who had prior infusion reactions.
- Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK1 receptor antagonist, as well as other drugs as indicated).
2.3 Dose Modifications for Adverse Reactions
Infusion-related Reactions
Slow or interrupt the infusion rate of TRODELVY if the patient develops an infusion-related reaction. Permanently discontinue TRODELVY for life-threatening infusion-related reactions [see Warnings and Precautions (5.3)]
Dose Modifications for Adverse Reactions
Withhold or discontinue TRODELVY to manage adverse reactions as described in Table 1. Do not re-escalate the TRODELVY dose after a dose reduction for adverse reactions has been made.
Table 1: Dose Modifications for Adverse Reactions Adverse Reaction Occurrence Dose Modification Severe Neutropenia [see Warnings and Precautions (5.1)] Grade 4 neutropenia ≥7 days,
OR
Grade 3 febrile neutropenia
(absolute neutrophil count <1000/mm3 and fever ≥38.5°C),
OR
At time of scheduled treatment, Grade 3-4 neutropenia which delays dosing by 2 or 3 weeks for recovery to ≤ Grade 1First 25% dose reduction and administer granulocyte-colony stimulating factor (G-CSF) Second 50% dose reduction Third Discontinue Treatment At time of scheduled treatment, Grade 3-4 neutropenia which delays dosing beyond 3 weeks for recovery to ≤ Grade 1 First Discontinue treatment Severe Non-Neutropenic Toxicity Grade 4 non-hematologic toxicity of any duration,
OR
Any Grade 3-4 nausea, vomiting or diarrhea due to treatment that is not controlled with antiemetics and anti-diarrheal agents [see Warnings and Precautions (5.2, 5.4)],
OR
Other Grade 3-4 non-hematologic toxicity persisting >48 hours despite optimal medical management,
OR
At time of scheduled treatment, Grade 3-4 non-neutropenic hematologic or non-hematologic toxicity, which delays dose by 2 or 3 weeks for recovery to ≤ Grade 1First 25% dose reduction Second 50% dose reduction Third Discontinue treatment In the event of Grade 3-4 non-neutropenic hematologic or non-hematologic toxicity, which does not recover to ≤ Grade 1 within 3 weeks First Discontinue treatment 2.4 Preparation for Administration
Reconstitution
- TRODELVY is a cytotoxic drug.
- Follow applicable special handling and disposal procedures1.
- Calculate the required dose (mg) of TRODELVY based on the patient's body weight at the beginning of each treatment cycle (or more frequently if the patient's body weight changed by more than 10% since the previous administration) [see Dosage and Administration (2.1)].
- Allow the required number of vials to warm to room temperature.
- Using a sterile syringe, slowly inject 20 mL of 0.9% Sodium Chloride Injection, USP, into each 180 mg TRODELVY vial. The resulting concentration will be 10 mg/mL.
- Gently swirl vials and allow to dissolve for up to 15 minutes. Do not shake. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be free of visible particulates, clear and yellow. Do not use the reconstituted solution if it is cloudy or discolored.
- Use immediately to prepare a diluted TRODELVY infusion solution.
Dilution
- Calculate the required volume of the reconstituted TRODELVY solution needed to obtain the appropriate dose according to patient's body weight. Withdraw this amount from the vial(s) using a syringe. Discard any unused portion remaining in the vial(s).
- Slowly inject the required volume of reconstituted TRODELVY solution into a polypropylene (PP) infusion bag, to minimize foaming. Do not shake the contents.
- Adjust the volume in the infusion bag as needed with 0.9% Sodium Chloride Injection, USP, to obtain a concentration of 1.1 mg/mL to 3.4 mg/mL (total volume should not exceed 500 mL). For patients whose body weight exceeds 170 kg, divide the total dosage of TRODELVY equally between two 500 mL infusion bags and infuse sequentially via slow infusion.
- Only 0.9% Sodium Chloride Injection, USP, should be used since the stability of the reconstituted product has not been determined with other infusion-based solutions. Use the diluted solution in the infusion bag immediately. If not used immediately, the infusion bag containing TRODELVY solution can be stored refrigerated 2°C to 8°C (36°F to 46°F) for up to 4 hours. After refrigeration, administer diluted solution within 4 hours (including infusion time).
Do Not Freeze or Shake. Protect from Light.
Administration
- Administer TRODELVY as an intravenous infusion. Protect infusion bag from light.
- An infusion pump may be used.
- Do not mix TRODELVY, or administer as an infusion, with other medicinal products.
- Upon completion of the infusion, flush the intravenous line with 20 mL 0.9% Sodium Chloride Injection, USP.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TRODELVY is contraindicated in patients who have experienced a severe hypersensitivity reaction to TRODELVY [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Neutropenia
TRODELVY can cause severe or life-threatening neutropenia. Withhold TRODELVY for absolute neutrophil count below 1500/mm3 on Day 1 of any cycle or neutrophil count below 1000/mm3 on Day 8 of any cycle. Withhold TRODELVY for neutropenic fever. Dose modifications may be required due to neutropenia [see Dosage and Administration (2.3)].
Febrile neutropenia occurred in 6% (24/408) patients treated with TRODELVY, including 8% (9/108) patients with mTNBC after at least two prior therapies. Less than 1% (1/408) of patients had febrile neutropenia leading to permanent discontinuation.
The incidence of Grade 1-4 neutropenia was 64% in patients with mTNBC (n=108). In all patients treated with TRODELVY (n=408), the incidence of Grade1-4 neutropenia was 54%; Grade 4 neutropenia occurred in 13%. Less than 1% (2/408) of patients permanently discontinued treatment due to neutropenia.
5.2 Diarrhea
TRODELVY can cause severe diarrhea. Withhold TRODELVY for Grade 3-4 diarrhea at the time of scheduled treatment administration and resume when resolved to ≤ Grade 1 [see Dosage and Administration (2.3)].
At the onset of diarrhea, evaluate for infectious causes and if negative, promptly initiate loperamide, 4 mg initially followed by 2 mg with every episode of diarrhea for a maximum of 16 mg daily. Discontinue loperamide 12 hours after diarrhea resolves. Additional supportive measures (e.g., fluid and electrolyte substitution) may also be employed as clinically indicated.
Patients who exhibit an excessive cholinergic response to treatment with TRODELVY (e.g., abdominal cramping, diarrhea, salivation, etc.) can receive appropriate premedication (e.g., atropine) for subsequent treatments.
Diarrhea occurred in 63% (68/108) of patients with mTNBC and 62% (254/408) of all patients treated with TRODELVY. In each population, events of Grade 3-4 occurred in 9% (10/108) of mTNBC patients and 9% (36/408) of all patients treated with TRODELVY. Four out of 408 patients (<1%) discontinued treatment because of diarrhea. Neutropenic colitis was observed in 2% (2/108) of patients in the mTNBC cohort and 1% of all patients treated with TRODELVY.
5.3 Hypersensitivity
TRODELVY can cause severe and life-threatening hypersensitivity. Anaphylactic reactions have been observed in clinical trials with TRODELVY.
Hypersensitivity reactions within 24 hours of dosing occurred in 37% (151/408) of patient treated with TRODELVY. Grade 3-4 hypersensitivity occurred in 1% (6/408) of patients treated with TRODELVY. The incidence of hypersensitivity reactions leading to permanent discontinuation of TRODELVY was 1% (3/408).
Pre-infusion medication for patients receiving TRODELVY is recommended. Observe patients closely for infusion-related reactions during each TRODELVY infusion and for at least 30 minutes after completion of each infusion [see Dosage and Administration (2.3)]. Medication to treat such reactions, as well as emergency equipment, should be available for immediate use.
5.4 Nausea and Vomiting
TRODELVY is emetogenic. Nausea occurred in 69% (74/108) of patients with mTNBC and 69% (281/408) of all patients treated with TRODELVY. Grade 3 nausea occurred in 6% (7/108) and 5% (22/408) of these populations, respectively.
Vomiting occurred in 49% (53/108) of patients with mTNBC and 45% (183/408) of all patients treated with TRODELVY. Grade 3 vomiting occurred in 6% (7/108) and 4% (16/408) of these patients, respectively.
Premedicate with a two or three drug combination regimen (e.g., dexamethasone with either a 5-HT3 receptor antagonist or an NK-1 receptor antagonist as well as other drugs as indicated) for prevention of chemotherapy-induced nausea and vomiting (CINV).
Withhold TRODELVY doses for Grade 3 nausea or Grade 3-4 vomiting at the time of scheduled treatment administration and resume with additional supportive measures when resolved to Grade ≤ 1 [see Dosage and Administration (2.3)].
Additional antiemetics and other supportive measures may also be employed as clinically indicated. All patients should be given take-home medications with clear instructions for prevention and treatment of nausea and vomiting.
5.5 Use in Patients with Reduced UGT1A1 Activity
Individuals who are homozygous for the uridine diphosphate-glucuronosyl transferase 1A1 (UGT1A1)*28 allele are at increased risk for neutropenia and may be at increased risk for other adverse reactions following initiation of TRODELVY treatment.
In 84% (343/408) of patients who received TRODELVY (up to 10 mg/kg on Days 1 and 8 of a 21-day cycle) and had retrospective UGT1A1 genotype results available, the incidence of Grade 4 neutropenia was 26% (10/39) in patients homozygous for the UGT1A1*28 allele, 13% (20/155) in patients heterozygous for the UGT1A1*28 allele and 11% (16/149) in patients homozygous for the wild-type allele [see Clinical Pharmacology (12.5)].
Closely monitor patients with reduced UGT1A1 activity for severe neutropenia. The appropriate dose for patients who are homozygous for UGT1A1*28 is not known and should be considered based on individual patient tolerance to treatment [see Dosage and Administration (2.3)].
5.6 Embryo-Fetal Toxicity
Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. TRODELVY contains a genotoxic component, SN-38, and targets rapidly dividing cells [see Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1)]. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with TRODELVY and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Neutropenia [see Warnings and Precautions (5.1)]
- Diarrhea [see Warnings and Precautions (5.2)]
- Hypersensitivity [see Warnings and Precautions (5.3)]
- Nausea and Vomiting [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described in the Warnings and Precautions section reflect exposure to TRODELVY as a single agent in a single-arm, open-label study (IMMU-132-01) in 408 patients with mTNBC and other malignancies who had received prior systemic therapeutic regimen for advanced disease. TRODELVY was administered as an intravenous infusion once weekly on Days 1 and 8 of 21-day treatment cycles at doses up to 10 mg/kg until disease progression or unacceptable toxicity.
The data in Table 2 reflect exposure to TRODELVY in a subset of 108 patients with mTNBC who had received at least two prior treatments for metastatic disease in study (IMMU-132-01). Patients received TRODELVY 10 mg/kg via intravenous infusion on Days 1 and 8 of 21-day treatment cycles until disease progression or unacceptable toxicity. The median treatment duration in these 108 patients was 5.1 months (range: 0-51 months).
Serious adverse reactions were reported in 31% of the patients. The most frequent serious adverse reactions (reported in >1%) of the patients receiving TRODELVY were febrile neutropenia (6%) vomiting (5%), nausea (3%), dyspnea (3%), diarrhea (4%), anemia (2%), pleural effusion, neutropenia, pneumonia, dehydration (each 2%).
TRODELVY was permanently discontinued for adverse reactions in 2% of patients. Adverse reactions leading to discontinuation were anaphylaxis, anorexia/fatigue, headache (each <1%, 1 patient for each event). Forty- five percent (45%) of patients experienced an adverse reaction leading to treatment interruption. The most common adverse reaction leading to treatment interruption was neutropenia (33%). Adverse reactions leading to dose reduction occurred in 33% of patients treated with TRODELVY, with 24% having one dose reduction and 9% with two dose reductions. The most common adverse reaction leading to dose reductions was neutropenia/febrile neutropenia.
Adverse reactions occurring in ≥10% of patients with mTNBC in the IMMU-132-01 study are summarized in Table 2.
Table 2: Adverse Reactions in ≥ 10% of Patients with mTNBC in IMMU-132-01 Adverse Reaction
TRODELVY
(n=108)Grade 1-4
(%)Grade 3-4
(%)Graded per NCI CTCAE v. 4.0
i. Including abdominal pain, distention, pain (upper), discomfort, tenderness
ii Including stomatitis, esophagitis, and mucosal inflammation
iii Including fatigue and asthenia
iv Including edema; and peripheral, localized, and periorbital edema
v Including rash; maculopapular, erythematous, generalized rash; dermatitis acneiform; skin disorder, irritation, and exfoliation
vi Including gait disturbance, hypoesthesia, muscular weakness, paresthesia, peripheral and sensory neuropathy
vii Including lower and upper respiratory tract infection, pneumonia, influenza, viral upper respiratory infection, bronchitis and respiratory syncytial virus infection
viii Includes cough and productive cough
ix Includes dyspnea and exertional dyspnea
Any adverse reaction 100 71 Gastrointestinal disorders 95 21 Nausea 69 6 Diarrhea 63 9 Vomiting 49 6 Constipation 34 1 Abdominal paini 26 1 Mucositisii 14 1 General disorders and administration site conditions 77 9 Fatigueiii 57 8 Edemaiv 19 0 Pyrexia 14 0 Blood and lymphatic system disorders 74 37 Neutropenia 64 43 Anemia 52 12 Thrombocytopenia 14 3 Metabolism and nutrition disorders 68 22 Decreased appetite 30 1 Hyperglycemia 24 4 Hypomagnesemia 21 1 Hypokalemia 19 2 Hypophosphatemia 16 9 Dehydration 13 5 Skin and subcutaneous tissue disorders 63 4 Alopecia 38 0 Rashv 31 3 Pruritus 17 0 Dry Skin 15 0 Nervous system disorders 56 4 Headache 23 1 Dizziness 22 0 Neuropathyvi 24 0 Dysgeusia 11 0 Infections and infestations 55 12 Urinary Tract Infection 21 3 Respiratory Infectionvii 26 3 Musculoskeletal and connective tissue disorders 54 1 Back pain 23 0 Arthralgia 17 0 Pain in extremity 11 0 Respiratory, thoracic and mediastinal disorders 54 5 Coughviii 22 0 Dyspneaix 21 3 Psychiatric disorders 26 1 Insomnia 13 0 Table 3: Laboratory Abnormalities observed in >10% of Patients while receiving TRODELVY Laboratory Abnormality TRODELVY
(n=108)All Grades
(%)Grade 3-4
(%)Hematology Decreased hemoglobin 93 6 Decreased leukocytes 91 26 Decreased neutrophils 82 32 Increased activated partial thromboplastin time 60 12 Decreased platelets 30 3 Chemistry Increased alkaline phosphatase 57 2 Decreased magnesium 51 3 Decreased calcium 49 3 Increased glucose 48 3 Increased aspartate aminotransferase 45 3 Decreased albumin 39 1 Increased alanine aminotransferase 35 2 Decreased potassium 30 3 Decreased phosphate 29 5 Decreased sodium 25 4.7 Increased magnesium 24 4 Decreased glucose 19 2 6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other sacituzumab govitecan products may be misleading.
The analysis of immunogenicity of TRODELVY in serum samples from 106 patients with mTNBC was evaluated using an electrochemiluminescence (ECL)-based immunoassay to test for anti-sacituzumab govitecan-hziy antibodies. Detection of the anti-sacituzumab govitecan-hziy antibodies was done using a 3-tier approach: screen, confirm, and titer. Persistent anti-sacituzumab govitecan-hziy antibodies developed in 2% (2/106) of patients.
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on TRODELVY
UGT1A1 Inhibitors
Concomitant administration of TRODELVY with inhibitors of UGT1A1 may increase the incidence of adverse reactions due to potential increase in systemic exposure to SN-38 [see Warning and Precaution (5.5) and Clinical Pharmacology (12.3, 12.5)]. Avoid administering UGT1A1 inhibitors with TRODELVY.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, TRODELVY can cause teratogenicity and/or embryo-fetal lethality when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. TRODELVY contains a genotoxic component, SN-38, and is toxic to rapidly dividing cells [see Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1)]. Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 – 4% and 15 – 20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of sacituzumab govitecan-hziy or SN-38 in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment and for 1 month after the last dose of TRODELVY.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of TRODELVY.
Contraception
Females
TRODELVY can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with TRODELVY and for 6 months after the last dose.
Infertility
Females
Based on findings in animals, TRODELVY may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of TRODELVY have not been established in pediatric patients.
8.5 Geriatric Use
Of the patients who received TRODELVY, 19/108 (18%) patients with mTNBC and 144/408 (35%) of all patients were ≥ 65 years old. No overall differences in safety and effectiveness were observed between these patients and younger patients.
8.6 Hepatic Impairment
No adjustment to the starting dose is required when administering TRODELVY to patients with mild hepatic impairment (bilirubin less than or equal to 1.5 ULN and AST/ALT < 3 ULN).
The exposure of TRODELVY in patients with mild hepatic impairment (bilirubin less than or equal to ULN and AST greater than ULN, or bilirubin greater than 1.0 to 1.5 ULN and AST of any level; n=12) was similar to patients with normal hepatic function (bilirubin or AST less than ULN; n=45).
The safety of TRODELVY in patients with moderate or severe hepatic impairment has not been established. TRODELVY has not been tested in patients with serum bilirubin > 1.5 ULN, or AST and ALT > 3 ULN, or AST and ALT > 5 ULN and associated with liver metastases.
No dedicated trial was performed to investigate the tolerability of TRODELVY in patients with moderate or severe hepatic impairment. No recommendations can be made for the starting dose in these patients.
- 10 OVERDOSAGE
-
11 DESCRIPTION
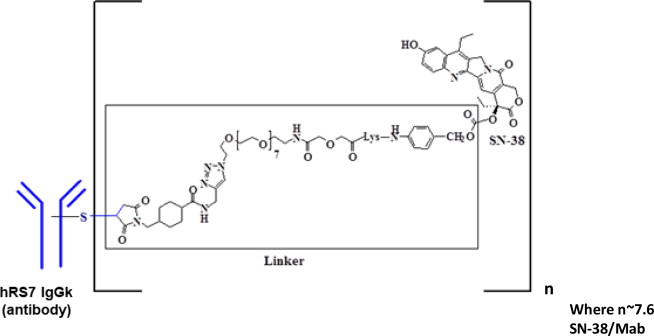
Sacituzumab govitecan-hziy is a Trop-2 directed antibody and topoisomerase inhibitor conjugate, composed of the following three components:
- the humanized monoclonal antibody, hRS7 IgG1ϰ (also called sacituzumab), which binds to Trop-2 (the trophoblast cell-surface antigen-2);
- the drug SN-38, a topoisomerase inhibitor;
- a hydrolysable linker (called CL2A), which links the humanized monoclonal antibody to SN-38.
The recombinant monoclonal antibody is produced by mammalian (murine myeloma) cells, while the small molecule components SN-38 and CL2A are produced by chemical synthesis. Sacituzumab govitecan-hziy contains on average 7 to 8 molecules of SN-38 per antibody molecule. Sacituzumab govitecan-hziy has a molecular weight of approximately 160 kilodaltons. Sacituzumab govitecan-hziy has the following chemical structure.
TRODELVY (sacituzumab govitecan-hziy) for injection is a sterile, preservative-free, off-white to yellowish lyophilized powder for intravenous use in a 50 mL clear glass single-dose vial, with a rubber stopper and crimp-sealed with an aluminum flip-off cap.
Each single-dose vial of TRODELVY delivers 180 mg sacituzumab govitecan-hziy, 77.3 mg 2-(N-morpholino) ethane sulfonic acid (MES), 1.8 mg polysorbate 80 and 154 mg trehalose dihydrate. Reconstitution with 20 mL of 0.9% Sodium Chloride Injection, USP, results in a concentration of 10 mg/mL with a pH of 6.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sacituzumab govitecan-hziy is a Trop-2-directed antibody-drug conjugate. Sacituzumab is a humanized antibody that recognizes Trop-2. The small molecule, SN-38, is a topoisomerase I inhibitor, which is covalently attached to the antibody by a linker. Pharmacology data suggest that sacituzumab govitecan-hziy binds to Trop-2-expressing cancer cells and is internalized with the subsequent release of SN-38 via hydrolysis of the linker. SN-38 interacts with topoisomerase I and prevents re-ligation of topoisomerase I-induced single strand breaks. The resulting DNA damage leads to apoptosis and cell death. Sacituzumab govitecan-hziy decreased tumor growth in mouse xenograft models of triple-negative breast cancer.
12.2 Pharmacodynamics
Exposure-response relationships and the time course of pharmacodynamics response are unknown for sacituzumab govitecan-hziy.
12.3 Pharmacokinetics
The serum pharmacokinetics of sacituzumab govitecan-hziy and SN-38 were evaluated in a study in a population of mTNBC patients who received sacituzumab govitecan-hziy as a single agent at a dose of 10 mg/kg. The pharmacokinetic parameters of sacituzumab govitecan-hziy and free SN-38 are presented in Table 4.
Table 4: Summary of Mean PK Parameters (±Standard Deviation) of Sacituzumab Govitecan-hziy and Free SN-38 Cmax: maximum plasma concentration
AUC0-168: area under plasma concentration curve through 168 hours
Sacituzumab govitecan-hziy Free SN-38 Cmax [ng/mL] 243,000 (±45,600) 127 (±60) AUC0-168 [h ng/mL] 5,210,000 (±1,230,000) 3,900 (±1,830) Elimination
The mean half-life of sacituzumab govitecan-hziy and free SN-38 was 16 and 18 hours, respectively. The clearance of the sacituzumab govitecan-hziy was 0.002 L/h/kg.
Metabolism
No metabolism studies with sacituzumab govitecan-hziy have been conducted. SN-38 (the small molecule moiety of sacituzumab govitecan-hziy) is metabolized via UGT1A1. The glucuronide metabolite of SN-38 (SN-38G) was detectable in the serum of patients.
Specific Populations
Pharmacokinetic analyses in a limited number of patients with mTNBC (n = 57) did not identify an effect of age or race on the pharmacokinetics of sacituzumab govitecan-hziy. Renal elimination is known to contribute minimally to the excretion of SN-38, the small molecule moiety of sacituzumab govitecan-hziy. There are no data on the pharmacokinetics of sacituzumab govitecan-hziy in patients with renal impairment or end-stage renal disease (CLcr ≤ 30 mL/min).
The exposure of sacituzumab govitecan-hziy is similar in patients with mild hepatic impairment (bilirubin less than or equal to ULN and AST greater than ULN, or bilirubin greater than 1.0 to less than 1.5 ULN and AST of any level; n=12) to patients with normal hepatic function (bilirubin or AST less than ULN; n=45).
Sacituzumab govitecan-hziy exposure is unknown in patients with moderate or severe hepatic impairment. SN-38 exposure may be elevated in such patients due to decreased hepatic UGT1A1 activity.
Drug Interaction Studies
No drug-drug interaction studies were conducted with sacituzumab govitecan-hziy or its components Inhibitors or inducers of UGT1A1 are expected to increase or decrease SN-38 exposure, respectively [see Drug Interactions (7)].
12.5 Pharmacogenomics
SN-38 is metabolized via UGT1A1 [see Clinical Pharmacology (12.3)]. Genetic variants of the UGT1A1 gene such as the UGT1A1*28 allele lead to reduced UGT1A1 enzyme activity. Individuals who are homozygous for the UGT1A1*28 allele are at increased risk for neutropenia from TRODELVY [see Warnings and Precautions (5.5)]. Approximately 20% of the Black or African American population, 10% of the White population, and 2% of the East Asian population are homozygous for the UGT1A1*28 allele. Decreased function alleles other than UGT1A1*28 may be present in certain populations.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with sacituzumab govitecan-hziy.
SN-38 was clastogenic in an in vitro mammalian cell micronucleus test in Chinese hamster ovary cells and was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay.
Fertility studies with sacituzumab govitecan-hziy have not been conducted. In a repeat-dose toxicity study in cynomolgus monkeys, intravenous administration of sacituzumab govitecan-hziy on Day 1 and Day 4 resulted in endometrial atrophy, uterine hemorrhage, increased follicular atresia of the ovary, and atrophy of vaginal epithelial cells at doses ≥ 60 mg/kg (≥ 6 times the human recommended dose of 10 mg/kg based on body weight).
-
14 CLINICAL STUDIES
The efficacy of TRODELVY was evaluated in study IMMU-132-01 (NCT01631552), a multicenter, single-arm, trial that enrolled 108 patients with metastatic triple-negative breast cancer (mTNBC) who had received at least two prior treatments for metastatic disease. Patients with bulky disease, defined as a mass >7 cm, were not eligible. Patients with treated brain metastases not receiving high dose steroids (>20 mg prednisone or equivalent) for at least four weeks were eligible. Patients with known Gilbert's disease were excluded.
Patients received TRODELVY 10 mg/kg intravenously on Days 1 and 8 of a 21-day treatment cycle. Patients were treated with TRODELVY until disease progression or intolerance to the therapy. Tumor imaging was obtained every 8 weeks, with confirmatory CT/MRI scans obtained 4-6 weeks after an initial partial or complete response, until progression requiring treatment discontinuation. Major efficacy outcome measures were investigator assessed overall response rate (ORR) using RECIST 1.1 and duration of response.
The median age was 55 years (range: 31 – 80 years); 87% of patients were younger than 65 years. The majority of patients were female (99%), and White (76%). At study entry, all patients had an ECOG performance status of 0 (29%) or 1 (71%). Seventy-six percent had visceral disease, 42% had hepatic metastases, 56% had lung/pleura metastases, and 2% had brain metastases. Twelve patients (11%) had Stage IV disease at the time of initial diagnosis.
The median number of prior systemic therapies received in the metastatic setting was 3 (range: 2 - 10). Prior chemotherapies in the metastatic setting included carboplatin or cisplatin (69%), gemcitabine (55%), paclitaxel or docetaxel (53%), capecitabine (51%), eribulin (45%), doxorubicin (24%), vinorelbine (16%), cyclophosphamide (19%), and ixabepilone (8%).
Overall, 98% of patients had received prior taxanes and 86% had received prior anthracyclines either in the (neo)adjuvant or metastatic setting.
Table 5 summarizes the efficacy results.
Table 5: Efficacy results for patients with mTNBC in IMMU-132-01 i investigator assessment
CI: confidence interval
+: denotes ongoing
TRODELVY
(N=108)Overall Response Rate i ORR (95% CI) 33.3% (24.6, 43.1) Complete response 2.8% Partial response 30.6% Response duration i Number of responders 36 Median, Months (95% CI) 7.7 (4.9, 10.8) Range, Months 1.9+, 30.4+ % with duration ≥ 6 months 55.6% % with duration ≥ 12 months 16.7% - 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TRODELVY (sacituzumab govitecan-hziy) for injection is a sterile, off-white to yellowish lyophilized powder in a single-dose vial. Each TRODELVY vial is individually boxed in a carton:
- NDC: 55135-132-01 contains one 180 mg vial
Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of reconstitution. Do not freeze.
TRODELVY is a cytotoxic drug. Follow applicable special handling and disposal procedures1.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Neutropenia
Advise patients of the risk of neutropenia. Instruct patients to immediately contact their healthcare provider if they experience fever, chills, or other signs of infection [see Warnings and Precautions (5.1)].
Diarrhea
Advise patients of the risk of diarrhea. Instruct patients to immediately contact their healthcare provider if they experience diarrhea for the first time during treatment; black or bloody stools; symptoms of dehydration such as lightheadedness, dizziness, or faintness; inability to take fluids by mouth due to nausea or vomiting; or inability to get diarrhea under control within 24 hours [see Warnings and Precautions (5.2)].
Hypersensitivity
Inform patients of the risk of serious infusion reactions and anaphylaxis. Instruct patients to immediately contact their healthcare provider if they experience facial, lip, tongue, or throat swelling, urticaria, difficulty breathing, lightheadedness, dizziness, chills, rigors, wheezing, pruritus, flushing, rash, hypotension or fever, that occur during or within 24 hours following the infusion [see Warnings and Precautions (5.3)].
Nausea/Vomiting
Advise patients of the risk of nausea and vomiting. Premedication according to established guidelines with a two or three drug regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) is also recommended. Additional antiemetics, sedatives, and other supportive measures may also be employed as clinically indicated. All patients should receive take-home medications for preventing and treating delayed nausea and vomiting, with clear instructions. Instruct patients to immediately contact their healthcare provider if they experience uncontrolled nausea or vomiting [see Warnings and Precautions (5.4)].
Embryo-Fetal Toxicity
Advise female patients to contact their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see Use in Specific Populations (8.1)].
Contraception
Advise female patients of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of TRODELVY [see Use in Specific Populations (8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of TRODELVY [see Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment and for 1 month after the last dose of TRODELVY [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that TRODELVY may impair fertility [see Use in Specific Populations (8.3)].
Manufactured by:
Immunomedics, Inc.
300 The American Road
Morris Plains, NJ 07950, USA
U.S. License No. 1737 -
PATIENT PACKAGE INSERT
The Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: April 2020
Patient Information
TRODELVYTM (troh-DELL-vee)
(sacituzumab govitecan-hziy) injectionWhat is the most important information I should know about TRODELVY?
TRODELVY can cause serious side effects, including:
-
Low white blood cell count (neutropenia). Low white blood cell counts are common with TRODELVY and can sometimes be severe and lead to infections that can be life-threatening. Your healthcare provider should check your blood cell counts during treatment with TRODELVY. If your white blood cell count is too low, your healthcare provider may need to lower your dose of TRODELVY, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection during treatment with TRODELVY:
- fever
- chills
- cough
- shortness of breath
- burning or pain when you urinate
-
Severe diarrhea. Diarrhea is common with TRODELVY and can also be severe. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control your diarrhea. If you lose too much body fluids (dehydration) your healthcare provider may need to give you fluids and electrolytes to replace body salts. If diarrhea happens later in your treatment, your healthcare provider may check you to see if the diarrhea may be caused by an infection. Your healthcare provider may decrease your dose or stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
Call your healthcare provider right away:- the first time that you get diarrhea during treatment with TRODELVY
- if you have black or bloody stools
- if you have symptoms of losing too much body fluid (dehydration) and body salts, such as lightheadedness, dizziness or faintness
- if you are unable to take fluids by mouth due to nausea or vomiting
- if you are not able to get your diarrhea under control within 24 hours
What is TRODELVY?
TRODELVY is a prescription medicine used to treat adults with breast cancer that is:
- estrogen and progesterone hormone receptor (HR) negative, and human epidermal growth factor receptor 2 (HER2)-negative (also called triple-negative breast cancer), and
- that has spread to other parts of the body metastatic, and
- who previously received at least two therapies for metastatic disease
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems.
It is not known if TRODELVY is safe and effective in children.Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure. Before receiving TRODELVY, tell your healthcare provider about all of your medical conditions, including if you:
- have been told that you carry a gene for uridine diphosphate-glucuronosyl transferase A1 (UGT1A1)*28. People who carry this gene have an increased risk of getting side effects with TRODELVY, especially low white blood cell counts. See “What is the most important information I should know about TRODELVY?”
- have liver problems.
- are pregnant or plan to become pregnant. TRODELVY can harm your unborn baby. Your healthcare provider should check to see if you are pregnant before you start receiving TRODELVY.
- Females who can become pregnant should use effective birth control during treatment and for 6 months after your last dose of TRODELVY. Talk to your healthcare provider about birth control choices that may be right for you during this time.
- Males with a female partner who can become pregnant should use effective birth control during treatment and for 3 months after your last dose of TRODELVY.
- Tell your healthcare provider right away if you or your partner become pregnant during treatment with TRODELVY.
- are breastfeeding or plan to breastfeed. It is not known if TRODELVY passes into your breastmilk and can harm your baby. Do not breastfeed during treatment and for 1 month after your last dose of TRODELVY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works. How will I receive TRODELVY?
- Your healthcare provider will give you TRODELVY into your vein through an intravenous (IV) line.
- TRODELVY is given 1 time each week, on Day 1 and on Day 8 of a 21-day treatment cycle.
- You will receive the first dose of TRODELVY over 3 hours. If you tolerate the first dose well, future doses may be given over 1 to 2 hours.
- Before each dose of TRODELVY, you will receive medicines to help prevent infusion reactions, and nausea and vomiting.
- You will be monitored for side effects during and for at least 30 minutes after you receive each infusion of TRODELVY.
- Your healthcare provider may slow down or temporarily stop your infusion of TRODELVY if you have an infusion-related reaction, or permanently stop TRODELVY if you have a life-threatening infusion-related reaction.
- Your healthcare provider will decide how long you will continue to receive TRODELVY.
What are the possible side effects of TRODELVY?
TRODELVY can cause serious side effects, including:
- See “What is the most important information I should know about TRODELVY?”
-
Severe and life-threatening allergic reactions. TRODELVY can cause severe and life-threatening allergic reactions during infusion (infusion-related reactions). Tell your healthcare provider or nurse right away if you get any of the following symptoms of an allergic reaction during an infusion of TRODELVY or within 24 hours after you receive a dose of TRODELVY:
- swelling of your face, lips, tongue, or throat
- hives
- skin rash or flushing of your skin
- difficulty breathing or wheezing
- lightheadedness, dizziness, feeling faint or pass out
- chills or shaking chills (rigors)
- fever
-
Nausea and vomiting. Nausea and vomiting are common with TRODELVY and can sometimes be severe. Before each dose of TRODELVY, you will receive medicines to help prevent nausea and vomiting. You should be given medicines to take home with you, along with instructions about how to take them to help prevent and treat any nausea and vomiting after you receive TRODELVY. Call your healthcare provider right away if you have nausea or vomiting that is not controlled with the medicines prescribed for you. Your healthcare provider may decide to decrease your dose or stop TRODELVY if your nausea and vomiting is severe and cannot be controlled with anti-nausea medicines.
The most common side effects of TRODELVY include:
- tiredness
- decreased red blood cell count
- hair loss
- constipation
- rash. See “Severe and life-threatening allergic reactions” above.
- decreased appetite
- stomach-area (abdomen) pain
TRODELVY may cause fertility problems in females, which could affect your ability to have a baby. Talk to your healthcare provider if fertility is a concern for you.
These are not all of the possible side effects of TRODELVY.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of TRODELVY.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about TRODELVY that is written for health professionals.What are the ingredients in TRODELVY?
Active ingredient: sacituzumab govitecan-hziy
Inactive ingredients: 2-(N-morpholino) ethane sulfonic acid (MES), polysorbate 80 and trehalose dihydrate
Manufactured by: Immunomedics, Inc., 300 The American Road, Morris Plains, NJ 07950, USA
U.S. License No. 1737
For more information about TRODELVY, go to www.TRODELVY.com or call 1-888-983-4668. -
Low white blood cell count (neutropenia). Low white blood cell counts are common with TRODELVY and can sometimes be severe and lead to infections that can be life-threatening. Your healthcare provider should check your blood cell counts during treatment with TRODELVY. If your white blood cell count is too low, your healthcare provider may need to lower your dose of TRODELVY, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection during treatment with TRODELVY:
-
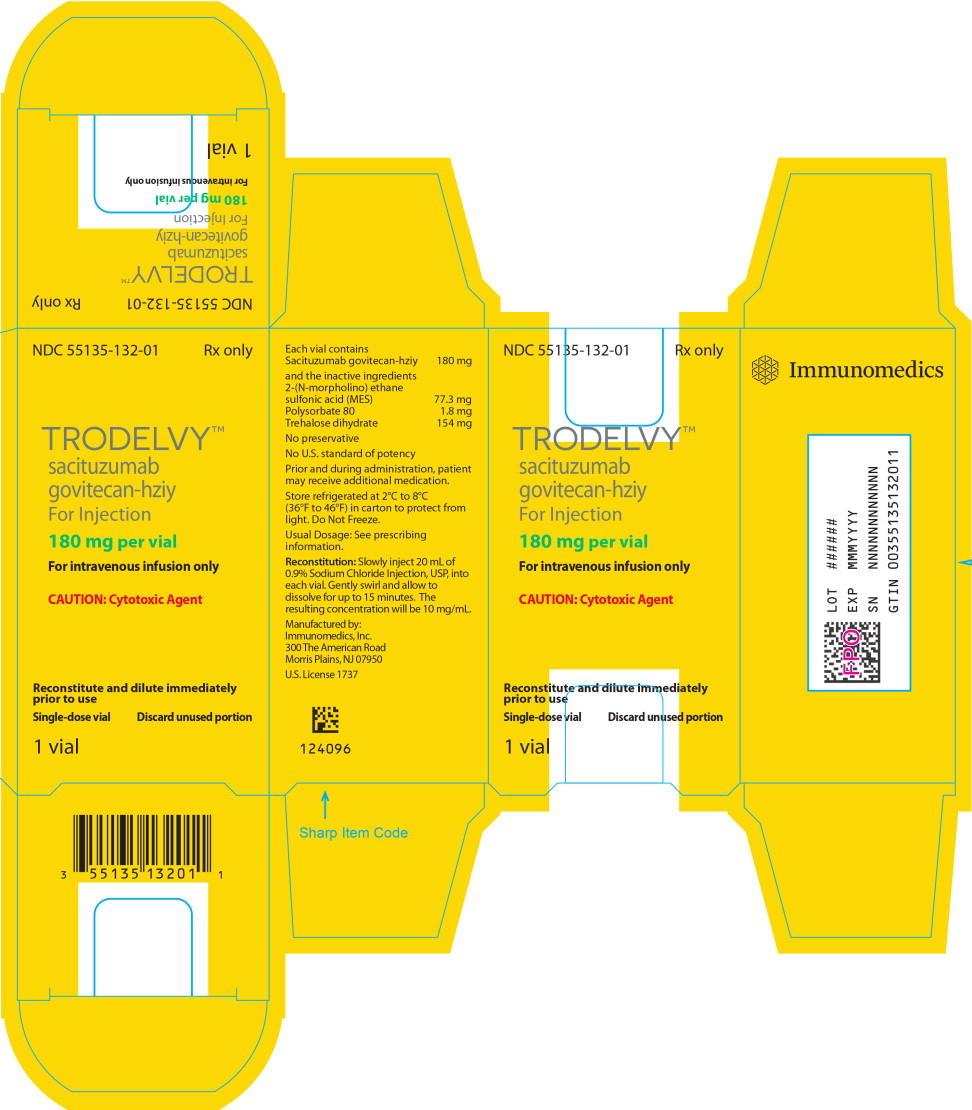
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 180 mg Box Label
NDC: 55135-132-01
Rx only
TRODELVY™
sacituzumab
govitecan-hziyFor Injection
180 mg per vial
For intravenous infusion only
CAUTION: Cytotoxic Agent
Reconstitute and dilute immediately
prior to useSingle-dose vial
Discard unused portion
1 vial
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 180 mg Vial Label
NDC: 55135-132-01
Rx only
TRODELVY™
sacituzumab
govitecan-hziyFor Injection
180 mg per vial
For intravenous infusion only
CAUTION: Cytotoxic Agent
Single-dose vial
Discard unused portion
1 vial
-
INGREDIENTS AND APPEARANCE
TRODELVY
sacituzumab govitecan powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55135-132 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SACITUZUMAB GOVITECAN (UNII: M9BYU8XDQ6) (Sacituzumab - UNII:73H2I6QS54) SACITUZUMAB GOVITECAN 180 mg Inactive Ingredients Ingredient Name Strength 2-(N-morpholino)ethanesulfonic acid (UNII: 2GNK67Q0C4) trehalose (UNII: B8WCK70T7I) polysorbate 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55135-132-01 1 in 1 BOX 04/23/2020 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761115 04/22/2020 Labeler - Immunomedics, Inc. (115350605)
Trademark Results [TRODELVY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRODELVY 90764802 not registered Live/Pending |
IMMUNOMEDICS, INC. 2021-06-09 |
 TRODELVY 90514477 not registered Live/Pending |
IMMUNOMEDICS, INC. 2021-02-05 |
 TRODELVY 88064964 not registered Live/Pending |
IMMUNOMEDICS, INC. 2018-08-03 |
 TRODELVY 87684433 not registered Live/Pending |
IMMUNOMEDICS, INC. 2017-11-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.