Ecolab Foaming AB HS by Kay Chemical Company Drug Facts
Ecolab Foaming AB HS by
Drug Labeling and Warnings
Ecolab Foaming AB HS by is a Otc medication manufactured, distributed, or labeled by Kay Chemical Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ECOLAB FOAMING AB HS- chloroxylenol liquid
Kay Chemical Company
----------
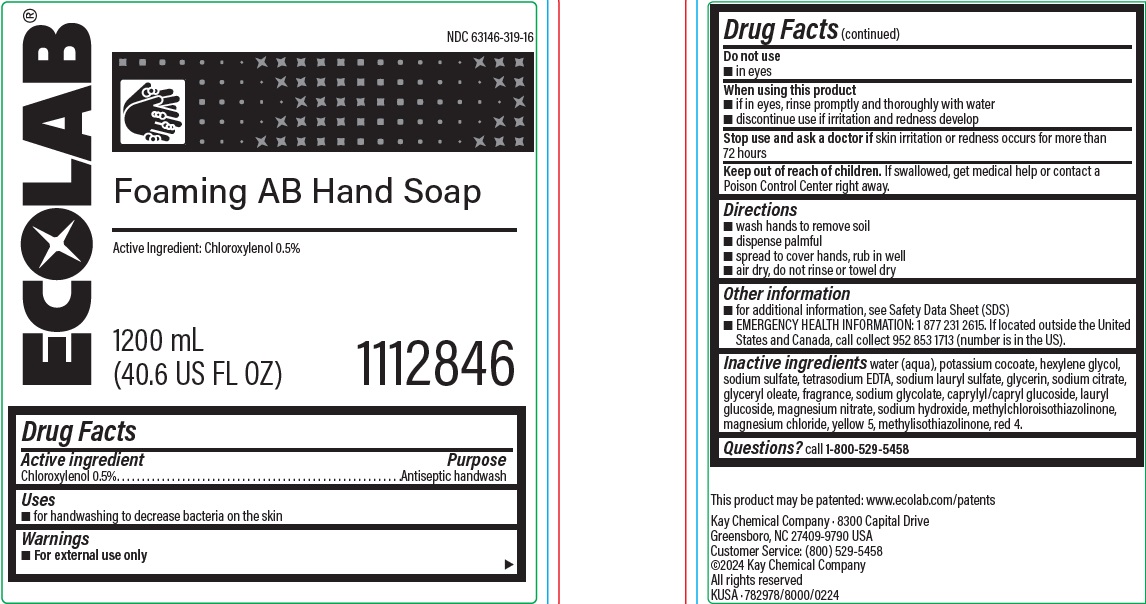
Drug Facts
Warnings
- For external use only
Directions
- wash hands to remove soil
- dispense palmful
- spread to cover hands, rub in well
- air dry, do not rinse or towel dry
Other information
- for additional infomration see Safety Data Sheet (SDS)
-
EMERGENCY HEALTH INFORMATION: 1 877 231 2615. If located outside the United States and Canada, call collect 952 853 1713 (number is in the US).
Inactive ingredients water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium edta, sodium lauryl sulfate, glycerin, sodium citrate, glyceryl oleate, fragrance, sodium glycolate, caprylyl/capryl glucoside, lauryl glucoside, magnesium nitrate, sodium hydroxide, methylchloroisothiazolinone, magnesium chloride, yellow 5, methylisothiazolinone, red 4
Principal display panel and representative label
ECOLAB
NDC: 63146-319-16
Foaming AB Hand Soap
Active Ingredient: Chloroxylenol 0.5%
1200 mL
(40.6 US FL OZ) 1112846
Kay Chemical Company · 8300 Capital Drive
Greensboro, NC 27409-9790 USA
Customer Service: (800) 529-5458
©2024 Kay Chemical Company
All rights reserved
KUSA · 782978/8000/0224
| ECOLAB FOAMING AB HS
chloroxylenol liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.