KEDBUMIN (albumin- human injection, solution
KEDBUMIN by
Drug Labeling and Warnings
KEDBUMIN by is a Other medication manufactured, distributed, or labeled by Kedrion S.p.A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KEDBUMIN® safely and effectively. See full prescribing information for KEDBUMIN® [Albumin (Human) U.S.P.] sterile, aqueous solution for single dose intravenous administration
Initial U.S. Approval: June, 2011.INDICATIONS AND USAGE
KEDBUMIN® is a 25% albumin solution indicated for:
- Hypovolemia (1.1) and Hypoalbuminemia (1.2)
- Prevention of central volume depletion after paracentesis due to cirrhotic ascites (1.3)
- Ovarian hyperstimulation syndrome (OHSS) (1.4)
- Adult Respiratory Distress Syndrome (ARDS) (1.5)
- Burns (1.6)
- Hemodialysis patients undergoing long term dialysis (1.7)
- Patients who cannot tolerate substantial volumes of salt solution (1.7)
- Priming as part of a cardiopulmonary bypass fluids (1.8)
DOSAGE AND ADMINISTRATION
Intravenous Administration Only. KEDBUMIN® may be diluted with 5% glucose or 0.9% sodium chloride. Concentration, dosage, and infusion-rate should be adjusted to the patient's individual requirements and indication (2.1). Daily dosage should not exceed 2g per kg body weight.
Indication: Dose Hypovolemia: Adults: Initial dose 25 g is suggested. Hypoalbuminemia: 50-75 g Prevention of Central Volume Depletion after Paracentesis due to Cirrhotic Ascites: Adults: 6-8 g for every 1000 mL of ascitic fluid removed OHSS: Adults: 50 to 100 g over 4 hours and repeated at 4-12 hour intervals as necessary. 10-50 g; single infusion ARDS: Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days if necessary Burns: Determined by direct observation of vital sign or measurement of either plasma oncotic pressure or protein content Hemodialysis: 100 mL Cardiopulmonary Bypass: Estimated from the difference between the desired and actual total serum protein concentration times the estimated plasma volume (approx 40mL per kg) times 2 DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- Severe anemia or heart failure in the presence of normal or increased intravascular volume (4).
- Hypersensitivity to albumin, the excipients, or components of the container.
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions include fever, chills, rash, nausea, vomiting, tachycardia, and hypotension (6).
To report SUSPECTED ADVERSE REACTIONS, contact KEDRION at 1-855-427-6378 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
1.1 Hypovolemia
1.2 Hypoalbuminemia
1.3 Prevention of Central Volume Depletion after Paracentesis due to Cirrhotic Ascites
1.4 Ovarian Hyperstimulation Syndrome (OHSS)
1.5 Adult Respiratory Distress Syndrome (ARDS)
1.6 Burns
1.7 Hemodialysis
1.8 Cardiopulmonary Bypass
2. DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Hypervolemia
5.3 Hemolysis
5.4 Large Volumes
5.5 Hydration
5.6 Infectious Diseases
6. ADVERSE REACTIONS
6.1 General
7. DRUG INTERACTION
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.4 Pediatric Use
10. OVERDOSE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
15. REFERENCES
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
1.1 Hypovolemia
For restoration and maintenance of circulating blood volume where volume deficiency is demonstrated and colloid use is appropriate.
1.2 Hypoalbuminemia
KEDBUMIN® is indicated for severe albumin deficiency caused by illness or active bleeding. When albumin deficiency results from excessive protein loss, the effect of albumin administration will be temporary unless the underlying disorder is reversed.
1.3 Prevention of Central Volume Depletion after Paracentesis due to Cirrhotic Ascites
KEDBUMIN® is indicated for maintenance of cardiovascular function following the removal of large volumes of ascitic fluid due to cirrhosis [1, 2].
1.4 Ovarian Hyperstimulation Syndrome (OHSS)
KEDBUMIN® is indicated as a plasma expander in the fluid management of severe forms of ovarian hyperstimulation syndrome (OHSS) [3, 4].
1.5 Adult Respiratory Distress Syndrome (ARDS)
KEDBUMIN® is indicated in conjunction with diuretics to correct fluid volume overload associated with ARDS [5].
1.6 Burns
KEDBUMIN® is indicated after > 24 hours post burn in patients experiencing severe albumin depletion in order to favor edema re-absorption [6].
-
2. DOSAGE AND ADMINISTRATION
Intravenous Administration Only.
2.1 Dosage
The concentration of the albumin preparation, dosage, and infusion-rate should be adjusted to the patient's individual requirements and indication.
Indication Dose Hypovolemia Adults: Initial dose of 25 g is suggested. Pediatric dosage should be adjusted based upon on age, weight and clinical conditions Hypoalbuminemia 50-75 g Prevention of Central Volume Depletion after Paracentesis due to Cirrhotic Ascites Adults: 6-8 g for every 1000 mL of ascitic fluid removed OHSS Adults: 50-100 g over 4 hours and repeated at 4-12 hour intervals as necessary. 10-50 g: single infusion ARDS Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days if necessary Burns The amount of albumin required to achieve adequate plasma volume and protein content should be determined by direct observation of vital signs or measurement of either plasma oncotic pressure or protein content Hemodialysis 100 mL Cardiopulmonary Bypass Required dose can be estimated from the difference between the desired and actual total serum protein concentration multiplied by the estimated plasma volume (approximately 40mL per kg) times 2 (to account for extravascular deficit, which absorbs about half of the administered dose) 2.2 Administration
Intravenous administration only.
Inspect visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Do not dilute with sterile water for injection as hemolysis may occur (5.3).
KEDBUMIN® may be diluted with 5% glucose or 0.9% sodium chloride.
Adjust the infusion rate to the rate of removal in plasma exchange.
Warm the product to room temperature if large volumes are to be administered.
Do not begin administration > 4 hours after the container has been entered. Discard unused material.
Record the batch number every time KEDBUMIN® is administered to a patient.
- 3. DOSAGE FORMS AND STRENGTHS
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity or allergic reactions have been observed, and may in some cases progress to severe anaphylaxis. Epinephrine should be available immediately to treat any acute hypersensitivity reaction.
5.2 Hypervolemia
KEDBUMIN® should be used with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk (10). Examples of such conditions may include but are not limited to:
- Heart failure
- Arterial hypertension
- Esophageal varices
- Pulmonary edema
- Hemorrhagic diathesis
- Severe anemia
- Renal and post-renal anuria
5.3 Hemolysis
Do not dilute KEDBUMIN® with Sterile Water for Injection, as this may cause hemolysis in recipients. There is a risk of potentially fatal hemolysis and acute renal failure from the use of Sterile Water for Injection as a diluent for 25% albumin 12. Suitable solutions for dilution include 5% glucose and 0.9% sodium chloride (2.2).
5.4 Large Volumes
When replacing comparatively large volumes of albumin, control of coagulation and hematocrit is essential. Ensure adequate substitution of other blood constituents as coagulation factors, electrolytes, platelets, and erythrocytes.
5.5 Hydration
The colloid osmotic effect of KEDBUMIN® 25% is approximately four times that of human blood. Therefore, when concentrated albumin is administered, ensure adequate hydration of the patient. Carefully monitor to guard against circulatory overload (10).
Hemodynamic performance should be monitored regularly. This may include arterial blood pressure and pulse rate, central venous pressure, pulmonary artery occlusion pressure, urine output, electrolyte levels, and hematocrit/hemoglobin.
5.6 Infectious Diseases
Albumin is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin.
ALL infections suspected by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Kedrion at 1-855-427-6378.
See also PATIENT COUNSELING INFORMATION (17).
-
6. ADVERSE REACTIONS
6.1 General
The most common adverse reactions include flushing, urticaria, fever, chills, nausea, vomiting, tachycardia and hypotension. These reactions normally disappear when the infusion rate is slowed or stopped.
If a severe reaction such as shock or anaphylaxis occurs, the infusion should be stopped and appropriate treatment initiated.
-
7. DRUG INTERACTION
KEDBUMIN® should not be mixed with other medicinal products including blood and blood components, but can be administered concomitantly with other parenterals such as whole blood, plasma, saline, glucose or sodium lactate when medically necessary.
KEDBUMIN® should not be mixed with protein hydrolysates or solutions containing alcohol since these combinations may cause protein precipitation.
-
8. USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
The adult dose may be given in children 12-16 years of age. Use of KEDBUMIN® in children less than 12 years of age has not been clinically evaluated. If administered to children the dosage will vary with the clinical state and body weight of the individual. Typically, a dose one-fourth to one-half the adult dose may be administered. The usual rate of administration in children should be one-fourth the adult rate. Physicians should weigh the risks and benefits of KEDBUMIN® in the pediatric population.
-
10. OVERDOSE
Hypervolemia may occur if the dosage and rate of infusion are too high. At the first clinical sign of circulatory overload, e.g. headache, dyspnea, jugular venous congestion, increased blood pressure, raised central venous pressure, or pulmonary edema, the infusion should be stopped and hemodynamic parameters carefully monitored. Additionally, diuresis or cardiac output should be increased in accordance with the severity of the clinical situation (5.2).
-
11. DESCRIPTION
KEDBUMIN® is a sterile, aqueous solution for single dose intravenous administration. The product contains 0.25 g per mL human albumin and is prepared by cold ethanol fractionation from pooled human plasma obtained from venous blood at FDA-licensed facilities located in the USA. Intermediate source material (albumin paste) is obtained from a U.S. licensed manufacturer. The colloid osmotic effect of KEDBUMIN® is approximately four times that of blood plasma.
KEDBUMIN® is a clear, slightly viscous liquid, with a yellow, amber, or green tint. The product is stabilized by the addition of 0.08 mmol sodium caprylate and 0.08 mmol sodium acetyltryptophan per gram of albumin. Additionally, each liter of material contains 130-160 mEq of sodium ion and ≤ 200 μg of aluminum. The product contains no preservatives.
KEDBUMIN® is heated for ten hours at 60°C. The KEDBUMIN® manufacturing process results in viral reduction in in vitro studies (see table below). These reductions are achieved through a combination of process steps including Cohn fractionation and final container heat treatment.
Mean Reduction Factor (log10) Enveloped viruses Non enveloped viruses Manufacturing Step HIV-1 BVDV PRV REO PPV HAV EMCV HIV-1: Human Immunodeficiency Virus Type 1 BVDV: Bovine Viral Diarrhoea Virus PRV: Pseudorabies Virus REO: Reovirus Type 3 PPV: Porcine Parvovirus HAV: Hepatitis A Virus EMCV: Encephalomyocarditis virus Fractionation of Effluent I to Effluent II +III 3.4 3.5 3.9 2.1 1.0 1.4 Fractionation of Effluent IV-1 to Effluent IV-4 3.7 Ethanol inactivation during fraction V precipitation > 3.71 > 3.38 Depth Filtration of Fraction V suspension 3.4 ≥ 3.4 4.9 4.2 2.0 Generation of Albumin paste 1.5 Heat treatment ≥ 6.06 > 5.17 > 5.07 4.62 > 5.0 Overall Reduction Factor ≥ 16.57 > 10.17 ≥ 15.75 11.62 5.2 > 8.4 3.7 -
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Human albumin is not a glycoprotein. It has the lowest molecular weight (66,241 Daltons) of all plasma proteins. Because of its three dimensional structure, solutions of albumin have a lower viscosity than solutions of other plasma proteins. This is important since work performed by the heart depends in part on the viscosity of blood. Human albumin accounts quantitatively for more than half of the total proteins in the circulation (by weight) and represents approximately 10 % of the protein synthesized in the liver.
Approximately 40% of albumin is contained in the circulation. The remainder is located in the extravascular space of tissues, principally muscle, skin, and intestine.
KEDBUMIN® 25% has a hyperoncotic effect.
A major function of albumin is its role in osmotic regulation. Albumin is responsible for 75% of normal oncotic pressure within the intravascular space [13, 14]. Other physiological functions include binding and transport of molecules (hormones, enzymes, drugs and toxins); free radical scavenging; hemostatic effects (platelet function inhibition and antithrombotic effects); and capillary membrane permeability [14].
12.3 Pharmacokinetics
Under normal conditions, the total exchangeable albumin pool is 4-5 g per kg body weight, of which 40-45% is present intravascularly and 55-60% is in the extravascular space. Increased capillary permeability will alter albumin kinetics and abnormal distribution may occur in conditions such as severe burns or septic shock.
Under normal conditions, the half-life of albumin is approximately 19 days. The balance between synthesis and breakdown is normally achieved by feed-back regulation. Elimination is predominantly intracellular and due to lysosomal proteases. In healthy subjects, less than 10% of infused albumin leaves the intravascular compartment during the first two hours following infusion. There is considerable individual variation in the effect on plasma volume. In some patients plasma volume can remain elevated for several hours. However, in critically ill patients, albumin can leak out of the vascular space in substantial amounts at an unpredictable rate.
-
15. REFERENCES
- 1.
Vermeulen LC, Jr., Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. Feb 27 1995;155(4):373-379.
- 2. Gines A, Fernandez-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. Oct 1996;111(4):1002-1010.
- 3. Aboulghar M, Evers JH, Al-Inany H. Intravenous albumin for preventing severe ovarian hyperstimulation syndrome: a Cochrane review. Hum Reprod. Dec 2002;17(12):3027-3032.
- 4. Medicine PCotASfR. Ovarian Hyperstimulation Syndrome. Fertility and Sterility. 2000;86(5 Suppl 1):S178-S183. .
- 5. Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. Aug 2005;33(8):1681-1687.
- 6. Manelli JC. [Is albumin administration useful in critical care for burnt patients?]. Ann Fr Anesth Reanim. 1996;15(4):507-513.
- 7. Tullis JL. Albumin. 2. Guidelines for clinical use. JAMA. Jan 31 1977;237(5):460-463 concl.
- 8. Schiff P. Albumin-containing plasma volume expanders. Aust N Z J Surg. Dec 1977;47(6):783-786.
- 9. Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. Aug 2001;72(2):527-533; discussion 534.
- 10. Gerety RJ, Aronson DL. Plasma derivatives and viral hepatitis. Transfusion. Sep-Oct 1982;22(5):347-351.
- 11. Gellis SS, Neefe JR, et al. Chemical, clinical, and immunological studies on the products of human plasma fractionation; inactivation of the virus of homologous serum hepatitis in solutions of normal human serum albumin by means of heat. J Clin Invest. Mar 1948;27(2):239-244.
- 12. Pierce LR, Gaines A, Finlayson JS, Varricchio F, Epstein JS. Hemolysis and acute renal failure due to the administration of albumin diluted in sterile water. Transfusion. Jan 1999;39(1):110-111.
- 13. Doweiko JP, Nompleggi DJ. Role of albumin in human physiology and pathophysiology. Jpen. Mar-Apr 1991;15(2):207-211.
- 14. Mendez CM, McClain CJ, Marsano LS. Albumin therapy in clinical practice. Nutr Clin Pract. Jun 2005;20(3):314-320.
- 2. Gines A, Fernandez-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. Oct 1996;111(4):1002-1010.
- 1.
Vermeulen LC, Jr., Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Arch Intern Med. Feb 27 1995;155(4):373-379.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
KEDBUMIN® is supplied as a sterile, aqueous solution for single dose intravenous administration containing 0.25 g per mL human albumin. It is available in the following glass vial size:
50 mL vial 25% (NDC: 76179-025-01) is packaged in one carton (NDC: 76179-025-02)
100 mL vial 25% (NDC: 76179-025-03) is packaged in one carton (NDC: 76179-025-04)
-
17. PATIENT COUNSELING INFORMATION
Inform patients being treated with KEDBUMIN® about the potential risks and benefits with its use (6). Discontinue immediately if allergic symptoms or circulatory overload occur (e.g. skin rashes, hives, itching, breathing difficulties, coughing, nausea, vomiting, fall in blood pressure, increased heart rate).
Inform patients that KEDBUMIN® is a derivative of human plasma and may contain infectious agents that cause disease (e.g., viruses, vCJD agent and theoretically, CJD agent). Inform patients that the risk that KEDBUMIN® may transmit an infectious agent has been reduced by screening plasma donors for prior exposure for certain viruses, by testing the donated plasma for certain virus infections, and by inactivating and/or removing certain viruses during manufacturing (5).
- SPL UNCLASSIFIED SECTION
-
PACKAGE LABEL
ALBUMIN(HUMAN) U.S.P.
KEDBUMIN 25% 12.5 g 50 mL
NDC: 7617902501
Rx only
Precautions: Do not freeze.
Instructions: The patient and physician should discuss the risks and benefits before using this product. For information on directions of administration, see the enclosed insert.
Contents:
Each 50 mL contains 12.5 g of Albumin (Human) in aqueous diluent. The solution is hyperoncotic. Sodium range 130-160 milliequivalents per liter.
Contains no preservative.
Heat treated at 60° C for 10 hours.
Distributed by:
Kedrion Biopharma, Inc. Parker Plaza,
400 Kelby Street Fort Lee, NJ 07024
United States
Manufactured by:
Kedrion S.p.A
55027
Bolognana, Gallicano
(Lucca) Italy
U.S. License No.1851
Keep the vial in the outer carton in order to protect from light. Store at temperatures not exceeding 30° C.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE THAN 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.
25%
8096036 - Rev. 02 03/12
Lot:
Expiration Date:
Lot:
Lot: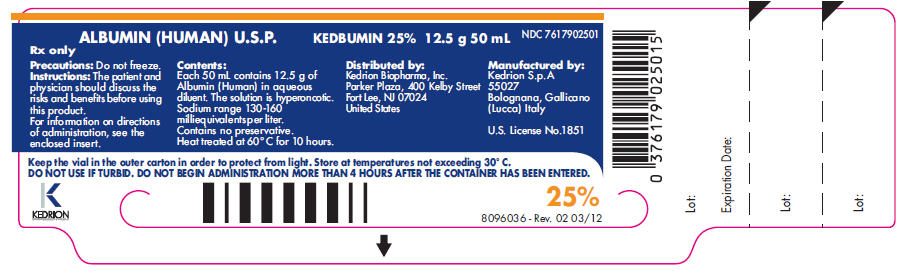
-
PRINCIPAL DISPLAY PANEL
NDC: 7617902502
Albumin (Human) U.S.P.
KEDBUMIN 25%
Solution 12.5 g 50 mL
Store at temperatures not exceeding 30° C
KEDRION
25%
Rx Only
Precautions:
Do not use KEDBUMIN 25% after the expiration date which is stated on the carton and label after "EXP." The expiration date refers to the last date of that month.
Do not store above 30°C.
Keep the vial stored in the outer carton in order to protect from light.
Do not freeze.
Instructions:
The patient and physician should discuss the risks and benefits before using this product. For information on directions of administration, see the enclosed insert.
DO NOT USE IF TURBID.
DO NOT BEING ADMINISTRATION 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.
Contents:
One 50 mL vial containing Albumin (Human) U.S.P. KEDBUMIN 25%
Each 50 mL contains 12.5 g of Albumin (Human) in aqueous diluent.
The solution is hyperoncotic.
Sodium range 130 – 160 milliequivalents per liter.
Stabilized with 0.08 millimole sodium caprylate and 0.08 millimole acetyltryptophan per gram of albumin
Contains no preservative
Heat treated at 60°C for 10 hours for viral inactivation
Distributed by:
Kedrion Biopharma, Inc.
Parker Plaza, 400 Kelby Street
Fort Lee, NJ 07024
United States
Manufactured by:
Kedrion S.p.A.
55027
Bolognana, Gallicano (Lucca) Italy
U.S. License No. 1851
Lot
Expiration Date:
55x47x88
8091354
Rev. 04 08/13
-
INGREDIENTS AND APPEARANCE
KEDBUMIN
albumin (human) injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 76179-025 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) (ALBUMIN HUMAN - UNII:ZIF514RVZR) ALBUMIN HUMAN 12.5 g in 50 mL Product Characteristics Color YELLOW (almost clear and colourless) , GREEN (almost clear and colourless) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76179-025-02 1 in 1 CARTON 1 NDC: 76179-025-01 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 2 NDC: 76179-025-04 1 in 1 CARTON 2 NDC: 76179-025-03 100 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125384 09/15/2011 Labeler - Kedrion S.p.A (339096023) Establishment Name Address ID/FEI Business Operations Kedrion S.p.A 339096023 MANUFACTURE(76179-025)
Trademark Results [KEDBUMIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KEDBUMIN 85330272 4321528 Live/Registered |
KEDRION BIOPHARMA INC. 2011-05-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.