TROMETHAMINE injection, solution
TROMETHAMINE by
Drug Labeling and Warnings
TROMETHAMINE by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Tromethamine Injection is a sterile, non-pyrogenic 0.3 M solution of tromethamine, adjusted to a pH of approximately 8.6 with glacial acetic acid. It is administered by intravenous injection, by addition to ACD blood for priming cardiac bypass equipment and by injection into the ventricular cavity during cardiac arrest.
Each 100 mL contains tromethamine 3.6 g (30 mEq) in water for injection. The solution is hypertonic 385 mOsmol/L (calc.). pH 8.6 (8.4-8.7).
The solution contains no bacteriostat, antimicrobial agent or added buffer (except acetic acid for pH adjustment) and is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
Tromethamine Injection is a parenteral systemic alkalizer and fluid replenisher.
Tromethamine, USP (sometimes called “tris” or “tris buffer”) is chemically designated 2-amino-2-(hydroxymethyl)-1, 3-propanediol, a solid readily soluble in water, also classified as an organic amine buffer. It has the following structural formula:
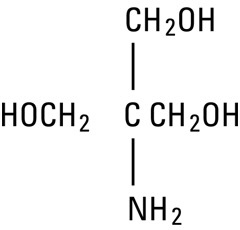
structural formula tromethamine
Water for Injection, USP is chemically designated H2O.
The EXCEL® plastic container is made from a multilayered film. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
Addition of medication should be accomplished using complete aseptic technique.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector and the other is a medication addition site. Refer to the Directions for Use of the container.
-
CLINICAL PHARMACOLOGY
When administered intravenously as a 0.3 M solution, tromethamine acts as a proton acceptor and prevents or corrects acidosis by actively binding hydrogen ions (H+). It binds not only cations of fixed or metabolic acids, but also hydrogen ions of carbonic acid, thus increasing bicarbonate anion (HCO3‾). Tromethamine also acts as an osmotic diuretic, increasing urine flow, urinary pH, and excretion of fixed acids, carbon dioxide and electrolytes. A significant fraction of tromethamine (30% at pH 7.40) is not ionized and therefore is capable of reaching equilibrium in total body water. This portion may penetrate cells and may neutralize acidic ions of the intracellular fluid.
The drug is rapidly eliminated by the kidney; 75% or more appears in the urine after eight hours. Urinary excretion continues over a period of three days.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
-
INDICATIONS AND USAGE
Tromethamine Injection is indicated for the prevention and correction of metabolic acidosis. In the following conditions it may help to sustain vital functions and thus provide time for treatment of the primary disease:
-
Metabolic Acidosis Associated with Cardiac Bypass Surgery.
Tromethamine Injection has been found to be primarily beneficial in correcting metabolic acidosis which may occur during or immediately following cardiac bypass surgical procedures. -
Correction of Acidity of ACD Blood in Cardiac Bypass Surgery.
It is well known that ACD blood is acidic and becomes more acidic on storage. Tromethamine effectively corrects this acidity. Tromethamine Injection may be added directly to the blood used to prime the pump-oxygenator. When ACD blood is brought to a normal pH range the patient is spared an initial acid load. Additional tromethamine may be indicated during cardiac bypass surgery should metabolic acidosis appear. -
Metabolic Acidosis Associated with Cardiac Arrest.
Acidosis is nearly always one of the consequences of cardiac arrest and, in some instances, may even be a causative factor in arrest. It is important therefore, that the correction of acidosis should be started promptly with other resuscitative efforts. By correcting acidosis, Tromethamine Injection has caused the arrested heart to respond to resuscitative efforts after standard methods alone had failed. In these cases, tromethamine was given intraventricularly. It is to be noted, however, that such precariously ill patients often have died subsequently of causes unrelated to the administration of tromethamine. With administration by the peripheral venous route, metabolic acidosis has been corrected in a majority of patients. The success in reinstitution of cardiac rhythm by this means probably has not been of the same order of magnitude as with the intraventricular route.
-
Metabolic Acidosis Associated with Cardiac Bypass Surgery.
- CONTRAINDICATIONS
-
WARNINGS
-
Large doses of Tromethamine Injection may depress ventilation, as a result of increased blood pH and reduced CO2 concentration. Thus, dosage should be adjusted so that blood pH is not allowed to increase above normal. In situations in which respiratory acidosis may be present concomitantly with metabolic acidosis, the drug may be used with mechanical assistance to ventilation.
-
Care must be exercised to prevent perivascular infiltration since this can cause inflammation, necrosis and sloughing of tissue. Venospasm and intravenous thrombosis, which may occur during infusion, can be minimized by insuring that the injection needle is well within the largest available vein and that solutions are slowly infused. Intravenous catheters are recommended. If perivascular infiltration occurs, institute appropriate countermeasures. (See ADVERSE REACTIONS).
-
Tromethamine Injection should be administered slowly and in amounts sufficient only to correct the existing acidosis, and to avoid overdosage and alkalosis. Overdosage in terms of total drug and/or too rapid administration, may cause hypoglycemia of a prolonged duration (several hours). Therefore, frequent blood glucose determinations should be made during and after therapy.
-
Extreme care should be exercised in patients with renal disease or reduced urinary output because of potential hyperkalemia and the possibility of a decreased excretion of tromethamine. In such patients, the drug should be used cautiously with electrocardiographic monitoring and frequent serum potassium determinations.
-
Because clinical experience has been limited generally to short-term use, the drug should not be administered for more than a period of one day except in a life-threatening situation.
The intravenous administration of Tromethamine Injection can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
-
-
PRECAUTIONS
-
Blood pH, PCO2 bicarbonate, glucose and electrolyte determinations should be performed before, during and after administration of Tromethamine Injection.
-
While it has not been shown that the drug increases coagulation time in humans, this possibility should be kept in mind since this has been noted experimentally in dogs.
Do not administer unless solution is clear and seal is intact. Discard unused portion.
Pregnancy:
Animal reproduction studies have not been conducted with tromethamine. It is also not known whether tromethamine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tromethamine should be given to a pregnant woman only if clearly needed.
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Tromethamine Injection is administered to a nursing mother.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Studies with Tromethamine Injection have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pediatric Use:
The safety and effectiveness of Tromethamine Injection in pediatric patients is based on over 30 years’ clinical experience documented in the literature and on safety surveillance. Tromethamine Injection has been used to treat severe cases of metabolic acidosis with concurrent respiratory acidosis because it does not raise PCO2 as bicarbonate does in neonates and infants with respiratory failure. It has also been used in neonates and infants with hypernatremia and metabolic acidosis to avoid the additional sodium given with the bicarbonate. However, because the osmotic effects of Tromethamine Injection are greater and large continuous doses are required, bicarbonate is preferred to Tromethamine Injection in the treatment of acidotic neonates and infants with RDS. Hypoglycemia may occur when this product is used in premature and even full-term neonates (see WARNINGS and ADVERSE REACTIONS).
Geriatric Use:
Clinical studies of Tromethamine Injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
-
ADVERSE REACTIONS
Generally, side effects have been infrequent.
Respiratory: Although the incidence of ventilatory depression is low, it is important to keep in mind that such depression may occur. Respiratory depression may be more likely to occur in patients who have chronic hypoventilation or those who have been treated with drugs which depress respiration. In patients with associated respiratory acidosis, tromethamine should be administered with mechanical assistance to ventilation.
Vascular: Extreme care should be taken to avoid perivascular infiltration. Local tissue damage and subsequent sloughing may occur if extravasation occurs. Chemical phlebitis and venospasm also have been reported.
Hematologic: Transient depression of blood glucose may occur.
Hepatic: Infusion via low-lying umbilical venous catheters has been associated with hepatocellular necrosis.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
Too rapid administration and/or excessive amounts of tromethamine may cause alkalosis, hypoglycemia, overhydration or solute overload. In the event of overdosage, discontinue the infusion, evaluate the patient and institute appropriate countermeasures (see WARNINGS, PRECAUTIONS and ADVERSE REACTIONS).
The LD50 values for the acute intravenous toxicity of Tromethamine Injection are influenced by the rate of infusion of the dose administered.
Intravenous LD50 Mice = 3,500 mg/kg
Intravenous LD50 Rats = 2,300 mg/kg
-
DOSAGE AND ADMINISTRATION
Tromethamine Injection is administered by slow intravenous infusion, by addition to pump-oxygenator ACD blood or other priming fluid or by injection into the ventricular cavity during cardiac arrest. For infusion by peripheral vein, a large needle should be used in the largest antecubital vein or an indwelling catheter placed in a large vein of an elevated limb to minimize chemical irritation of the alkaline solution during infusion. Catheters are recommended.
Dosage and rate of administration should be carefully supervised to avoid overtreatment (alkalosis). Pretreatment and subsequent determinations of blood values (e.g. pH, PCO2, PO2, glucose and electrolytes) and urinary output should be made as necessary to monitor dosage and progress of treatment. In general, dosage should be limited to an amount sufficient to increase blood pH to normal limits (7.35 to 7.45) and to correct acid-base derangements. The total quantity to be administered during the period of illness will depend upon the severity and progression of the acidosis. The possibility of some retention of tromethamine, especially in patients with impaired renal function, should be kept in mind.
The intravenous dosage of Tromethamine Injection may be estimated from the buffer base deficit of the extracellular fluid in mEq/liter determined by means of the Siggaard-Andersen nomogram. The following formula is intended as a general guide:
Tromethamine Injection (mL of 0.3 M) Required =
Body Weight (kg) X
Base Deficit (mEq/liter) X 1.1*
Thus, a 70 kg patient with a buffer base deficit (“negative base excess”) of 5 mEq/liter would require 70 x 5 x 1.1 = 385 mL of Tromethamine Injection containing 13.9 g (115 mEq) of tromethamine. The need for administration of additional Tromethamine Injection is determined by serial determinations of the existing base deficit.
* Factor of 1.1 accounts for an approximate reduction of 10% in buffering capacity due to the presence of sufficient acetic acid to lower pH of the 0.3 M solution to approximately 8.6.
Correction of Metabolic Acidosis Associated with Cardiac Bypass Surgery: An adverse dose of approximately 9.0 mL/kg (324 mg/kg) has been used in clinical studies with Tromethamine Injection. This is equivalent to a total dose of 630 mL (189 mEq) for 70 kg patient. A total single dose of 500 mL (150 mEq) is considered adequate for most adults. Larger single doses (up to 1000 mL) may be required in unusually severe cases.
It is recommended that individual doses should not exceed 500 mg/kg (227 mg/lb) over a period of not less than one hour. Thus, for a 70 kg (154 pound) patient the dose should not exceed a maximum of 35 g per hour (1078 mL of a 0.3 M solution). Repeated determinations of pH and other clinical observations should be used as a guide to the need for repeat doses.
Correction of Acidity of ACD Blood in Cardiac Bypass Surgery: The pH of stored blood ranges from 6.80 to 6.22 depending upon the duration of storage. The amount of Tromethamine Injection used to correct this acidity ranges from 0.5 to 2.5 g (15 to 77 mL of a 0.3 M solution) added to each 500 mL of ACD blood used for priming the pump-oxygenator. Clinical experience indicates that 2 g (62 mL of a 0.3 M solution) added to 500 mL of ACD blood is usually adequate.
Correction of Metabolic Acidosis Associated with Cardiac Arrest: In the treatment of cardiac arrest, Tromethamine Injection should be given at the same time that other standard resuscitative measures, including manual systole, are being applied. If the chest is open, Tromethamine Injection is injected directly into the ventricular cavity. From 2 to 6 g (62 to 185 mL of a 0.3 M solution) should be injected immediately. Do not inject into the cardiac muscle.
If the chest is not open, from 3.6 to 10.8 g (111 to 333 mL of a 0.3 M solution) should be injected immediately into a larger peripheral vein. Additional amounts may be required to control acidosis persisting after cardiac arrest is reversed.
Correction of Metabolic Acidosis Associated with RDS in Neonates and Infants: The initial dose of Tromethamine Injection should be based on initial pH and birthweight amounting to approximately 1 mL per kg for each pH unit below 7.4. Further doses have been given according to changes in PaO2, pH and PCO2.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit (see CONTRAINDICATIONS).
-
HOW SUPPLIED
Tromethamine Injection 18 g/500 mL (3.6 g/100 mL) is supplied in sterile and nonpyrogenic 500 mL (150 mEq) single-dose EXCEL® flexible containers with clear overwrap, 24 per case.
Not made with natural rubber latex, PVC or DEHP.
NDC REF Size 0264-8031-10 P8031 500 mL Store at 20 to 25°C (68 to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
Protect from freezing.
Initiated: December 2024
EXCEL® is a registered trademark of B. Braun Medical Inc.
-
Directions for Use of EXCEL® Container
Caution: Do not use plastic containers in series connection.
To Open
Tear overwrap down at notch and remove solution container. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired.
NOTE: Before use, perform the following checks:
Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter.
Any container which is suspect should not be used.
Use only if solution is clear and container and seals are intact.Preparation for Administration
1. Remove plastic protector from sterile set port at bottom of container.
2. Attach administration set. Refer to complete directions accompanying set.To Add Medication
Warning: Some additives may be incompatible.
To Add Medication Before Solution Administration
1. Prepare medication site.
2. Using syringe with 18–22 gauge needle, puncture medication port and inner diaphragm and inject.
3. Squeeze and tap ports while ports are upright and mix solution and medication thoroughly.To Add Medication During Solution Administration
1. Close clamp on the set.
2. Prepare medication site.
3. Using syringe with 18–22 gauge needle of appropriate length (at least 5/8 inch), puncture resealable medication port and inner diaphragm and inject.
4. Remove container from IV pole and/or turn to an upright position.
5. Evacuate both ports by tapping and squeezing them while container is in the upright position.
6. Mix solution and medication thoroughly.Return container to in-use position and continue administration
- SPL UNCLASSIFIED SECTION
-
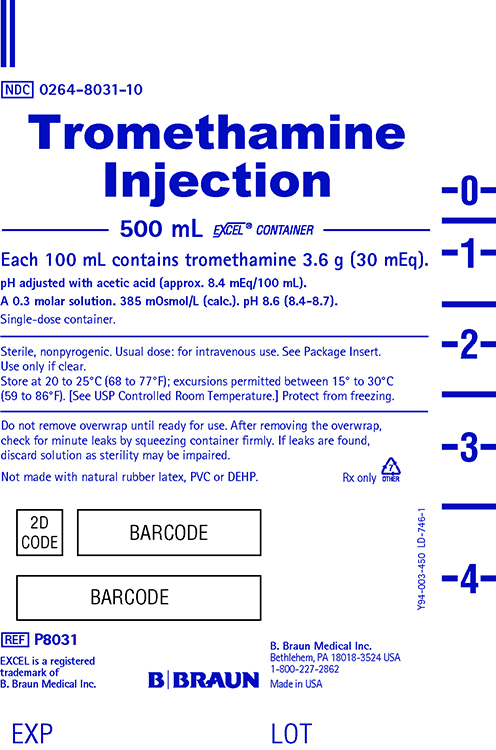
PRINCIPAL DISPLAY PANEL - 500 ML EXCEL® Container Label
NDC 0264-8031-10
Tromethamine Injection
500 mL EXCEL® Container
Each 100 mL contains tromethamine 3.6 g (30 mEq).
pH adjusted with acetic acid (approx. 8.4 mEq/100 mL).
A 0.3 molar solution. 385 mOsmol/L (calc.). pH 8.6 (8.4-8.7).Single-dose container.
Sterile, nonpyrogenic. Usual dose: for intravenous use. See Package Insert.
Use only if clear.
Store at 20 to 25°C (68 to 77°F); excursions permitted between 15° to 30°C
(59 to 86°F). [See USP Controlled Room Temperature.] Protect from freezing.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only
Y94-003-450 LD-746-1
REF P8031
EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Made in USAEXP
LOT
LD-746-1
-
INGREDIENTS AND APPEARANCE
TROMETHAMINE
tromethamine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-8031 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TROMETHAMINE (UNII: 023C2WHX2V) (TROMETHAMINE - UNII:023C2WHX2V) TROMETHAMINE 3.6 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ACETIC ACID (UNII: Q40Q9N063P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-8031-10 24 in 1 CASE 08/27/2025 1 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211558 08/27/2025 Labeler - B. Braun Medical Inc. (002397347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.