Gabapentin by Avera McKennan Hospital GABAPENTIN capsule
Gabapentin by
Drug Labeling and Warnings
Gabapentin by is a Prescription medication manufactured, distributed, or labeled by Avera McKennan Hospital. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GABAPENTIN CAPSULES safely and effectively. See full prescribing information for GABAPENTIN CAPSULES.
GABAPENTIN capsules for oral use
Initial U.S. Approval: 1993
RECENT MAJOR CHANGES
- Warnings and Precautions: Anaphylaxis and Angioedema: discontinue Gabapentin and evaluate patient immediately (5.2) 9/2015
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
· Postherpetic Neuralgia (2.1)
o Dose can be titrated up as needed to a dose of 1800 mg/day
o Day 1: Single 300 mg dose
o Day 2: 600 mg/day (i.e., 300 mg two times a day)
o Day 3: 900 mg/day (i.e., 300 mg three times a day)
· Epilepsy with Partial Onset Seizures (2.2)
o Patients 12 years of age and older: starting dose is 300 mg three times daily; may be titrated up to 600 mg three times daily
o Patients 3 to 11 years of age: starting dose range is 10 to 15 mg/kg/day, given in three divided doses; recommended dose in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses; the recommended dose in patients 5 to 11 years of age is 25 to 35 mg/kg/day, given in three divided doses. The recommended dose is reached by upward titration over a period of approximately 3 days
· Dose should be adjusted in patients with reduced renal function (2.3, 2.4)
DOSAGE FORMS AND STRENGTHS
· Capsules: 100 mg, 300 mg, and 400 mg (3)
CONTRAINDICATIONS
Known hypersensitivity to gabapentin or its ingredients (4)
WARNINGS AND PRECAUTIONS
· Drug Reaction with Eosinophilia and Systemic Symptoms (Multiorgan hypersensitivity): discontinue gabapentin if an alternative etiology cannot be established (5.1)
· Anaphylaxis and Angioedema: discontinue gabapentin and evaluate patient immediately (5.2)
· Driving impairment: warn patients not to drive until they have gained sufficient experience with gabapentin to assess whether it will impair their ability to drive (5.3)
· Somnolence/Sedation and Dizziness: gabapentin may impair the patient’s ability to operate complex machinery (5.4)
· Increased seizure frequency may occur in patients with seizure disorders if gabapentin is abruptly discontinued (5.5)
· Suicidal Behavior and Ideation: monitor for suicidal thoughts and behavior (5.6)
· Neuropsychiatric Adverse Reactions in Children 3-12 Years of Age: monitor for such events (5.7)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥8% and at least twice that for placebo) were:
· Postherpetic neuralgia: dizziness, somnolence, and peripheral edema (6.1)
· Epilepsy in patients >12 years of age: somnolence, dizziness, ataxia, fatigue, and nystagmus (6.1)
· Epilepsy in patients 3 to 12 years of age: viral infection, fever, nausea and/or vomiting, somnolence, and hostility (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ascend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901)or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
2 DOSAGE & ADMINISTRATION
2.1 Dosage for Postherpetic Neuralgia
2.2 Dosage for Epilepsy with Partial Onset Seizures
2.3 Dosage Adjustment in Patients with Renal Impairment
2.4 Dosage in Elderly
2.5 Administration Information
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
5.2 Anaphylaxis and Angioedema
5.3 Effects on Driving and Operating Heavy Machinery
5.4 Somnolence/Sedation and Dizziness
5.5 Withdrawal Precipitated Seizure, Status Epilepticus
5.6 Suicidal Behavior and Ideation
5.7 Neuropsychiatric Adverse Reactions (Pediatric Patients 3 to 12 Years of Age)
5.8 Tumorigenic Potential
5.9 Sudden and Unexplained Death in Patients with Epilepsy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Other Antiepileptic Drugs
7.2 Opioids
7.3 Maalox® (aluminum hydroxide, magnesium hydroxide)
7.4 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
14 CLINICAL STUDIES
14.1 Postherpetic Neuralgia
14.2 Epilepsy for Partial Onset Seizures (Adjunctive Therapy)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE
-
2 DOSAGE & ADMINISTRATION
2.1 Dosage for Postherpetic Neuralgia
In adults with postherpetic neuralgia, gabapentin may be initiated on Day 1 asa single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and onDay 3 as 900 mg/day (300 mg three times a day). The dose can subsequently betitrated up as needed for pain relief to a dose of 1800 mg/day (600 mg threetimes a day). In clinical studies, efficacy was demonstrated over a range ofdoses from 1800 mg/day to 3600 mg/day with comparable effects across the doserange; however, in these clinical studies, the additional benefit of usingdoses greater than 1800 mg/day was not demonstrated.
2.2 Dosage for Epilepsy with Partial Onset Seizures
Patients 12years of age and above
The startingdose is 300 mg three times a day. The recommended maintenance dose of gabapentin is 300 mg to 600 mg threetimes a day. Dosages up to 2400 mg/day have been well tolerated in long-termclinical studies. Doses of 3600 mg/day have also been administered to a smallnumber of patients for a relatively short duration, and have been welltolerated. Administer gabapentinthree times a day using 300 mg or 400 mg capsules. The maximum time betweendoses should not exceed 12 hours.
PediatricPatients Age 3 to 11 years
The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in threedivided doses, and the recommended maintenance dose reached by upward titrationover a period of approximately 3 days. The recommended maintenance dose of gabapentin in patients 3 to 4 years ofage is 40 mg/kg/day, given in three divided doses. The recommended maintenancedose of gabapentin in patients 5to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divideddoses. Gabapentin may beadministered as the oral capsule. Dosages up to 50 mg/kg/day have been welltolerated in a long-term clinical study. The maximum time interval betweendoses should not exceed 12 hours.
2.3 Dosage Adjustment in Patients with Renal Impairment
Dosageadjustment in patients 12 years of age and older with renal impairment orundergoing hemodialysis is recommended, as follows (see dosing recommendationsabove for effective doses in each indication):
TABLE 1. Gabapentin Dosage Based on Renal Function
Renal Function
Creatinine Clearance
(mL/min)
Total Daily
Dose Range
(mg/day)
Dose Regimen (mg)
≥ 60
900 to 3600
300 TID 400 TID 600 TID
800 TID 1200 TID
> 30 to 59
400 to 1400
200 BID 300 BID 400 BID
500 BID 700 BID
> 15 to 29
200 to 700
200 QD 300 QD 400 QD
500 QD 700 QD
15a
100 to 300
100 QD 125 QD 150 QD
200 QD 300 QD
Post-Hemodialysis Supplemental Dose (mg)b
Hemodialysis
125b 150b 200b 250b 350b
TID = Threetimes a day; BID = Two times a day; QD = Single daily dose
a For patients with creatinine clearance <15mL/min, reduce daily dose in proportion to creatinine clearance (e.g., patientswith a creatinine clearance of 7.5 mL/min should receive one-half the dailydose that patients with a creatinine clearance of 15 mL/min receive).
b Patients on hemodialysis should receivemaintenance doses based on estimates of creatinine clearance as indicated inthe upper portion of the table and a supplemental post-hemodialysis doseadministered after each 4 hours of hemodialysis as indicated in the lowerportion of the table.
Creatinine clearance (CLCr) is difficult to measure in outpatients. Inpatients with stable renal function, creatinine clearance can be reasonablywell estimated using the equation of Cockcroft and Gault:
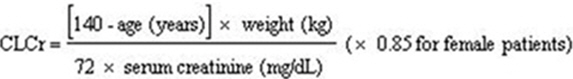
The use of gabapentinin patients less than 12 years of age with compromised renal function has notbeen studied.
2.4 Dosage in Elderly
Because elderly patients are more likely to havedecreased renal function, care should be taken in dose selection, and doseshould be adjusted based on creatinine clearance values in these patients.
2.5 Administration Information
Administer gabapentin orally with or without food.
Gabapentin capsules should be swallowedwhole with water.
If the gabapentin dose isreduced, discontinued, or substituted with an alternative medication, thisshould be done gradually over a minimum of 1 week (a longer period may beneeded at the discretion of the prescriber).
- 3 DOSAGE FORMS & STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Drug Reactionwith Eosinophilia and Systemic Symptoms (DRESS), also known as multiorganhypersensitivity, has occurred with gabapentin. Some of these reactions havebeen fatal or life-threatening. DRESS typically, although not exclusively,presents with fever, rash, and/or lymphadenopathy, in association with otherorgan system involvement, such as hepatitis, nephritis, hematologicalabnormalities, myocarditis, or myositis sometimes resembling an acute viralinfection. Eosinophilia is often present. This disorder is variable in itsexpression, and other organ systems not noted here may be involved.
It is important to note that early manifestations of hypersensitivity,such as fever or lymphadenopathy, may be present even though rash is notevident. If such signs or symptoms are present, the patient should be evaluated immediately. Gabapentin shouldbe discontinued if an alternative etiology for the signs or symptoms cannot beestablished.
5.2 Anaphylaxis and Angioedema
Gabapentin can cause anaphylaxis and angioedema after the first dose or at anytime during treatment. Signs and symptoms in reported cases have includeddifficulty breathing, swelling of the lips, throat, and tongue, and hypotensionrequiring emergency treatment. Patients should be instructed to discontinue gabapentin and seek immediate medical care should theyexperience signs or symptoms of anaphylaxis or angioedema.
5.3 Effects on Driving and Operating Heavy Machinery
Patients taking gabapentin should not drive until they have gained sufficient experience to assess whether gabapentin impairs their ability to drive. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients’ ability to assess their own driving competence, as well as their ability to assess the degree of somnolence caused by gabapentin, can be imperfect. The duration of driving impairment after starting therapy with gabapentin is unknown. Whether the impairment is related to somnolence [see Warnings and Precautions (5.4 )] or other effects of gabapentin is unknown.
Moreover, because gabapentin causes somnolence and dizziness [see Warnings and Precautions (5.4)], patients should be advised not to operate complex machinery until they have gained sufficient experience on gabapentin to assess whether gabapentin impairs their ability to perform such tasks.
5.4 Somnolence/Sedation and Dizziness
During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for dizziness, and 13% in drug versus 6% in placebo for ataxia. In these trials somnolence, ataxia and fatigue were common adverse reactions leading to discontinuation of gabapentin in patients older than 12 years of age, with 1.2%, 0.8% and 0.6% discontinuing for these events, respectively.
During the controlled trials in patients with post-herpetic neuralgia, somnolence and dizziness were reported at a greater rate compared to placebo in patients receiving gabapentin, in dosages up to 3600 mg per day: i.e., 21% in gabapentin-treated patients versus 5% in placebo-treated patients for somnolence and 28% in gabapentin-treated patients versus 8% in placebo-treated patients for dizziness. Dizziness and somnolence were among the most common adverse reactions leading to discontinuation of gabapentin.
Patients should be carefully observed for signs of central nervous system (CNS) depression, such as somnolence and sedation, when gabapentin is used with other drugs with sedative properties because of potential synergy. In addition, patients who require concomitant treatment with morphine may experience increases in gabapentin concentrations and may require dose adjustment [see Drug Interactions (7.2)].
5.5 Withdrawal Precipitated Seizure, Status Epilepticus
Antiepilepticdrugs should not be abruptly discontinued because of the possibility ofincreasing seizure frequency.
In the placebo-controlled epilepsy studies in patients >12 years ofage, the incidence of status epilepticus in patients receiving gabapentin was0.6% (3 of 543) vs. 0.5% in patients receiving placebo (2 of 378). Among the2074 patients >12 years of age treated with gabapentin across all epilepsystudies (controlled and uncontrolled), 31 (1.5%) had status epilepticus. Ofthese, 14 patients had no prior history of status epilepticus either beforetreatment or while on other medications. Because adequate historical data arenot available, it is impossible to say whether or not treatment with gabapentinis associated with a higher or lower rate of status epilepticus than would beexpected to occur in a similar population not treated with gabapentin.
5.6 Suicidal Behavior and Ideation
Antiepilepticdrugs (AEDs), including gabapentin, increase the risk of suicidal thoughts orbehavior in patients taking these drugs for any indication. Patients treatedwith any AED for any indication should be monitored for the emergence orworsening of depression, suicidal thoughts or behavior, and/or any unusualchanges in mood or behavior.
Pooled analysesof 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11different AEDs showed that patients randomized to one of the AEDs hadapproximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) ofsuicidal thinking or behavior compared to patients randomized to placebo. Inthese trials, which had a median treatment duration of 12 weeks, the estimatedincidence rate of suicidal behavior or ideation among 27,863 AED-treatedpatients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients,representing an increase of approximately one case of suicidal thinking orbehavior for every 530 patients treated. There were four suicides indrug-treated patients in the trials and none in placebo-treated patients, butthe number is too small to allow any conclusion about drug effect on suicide.
The increasedrisk of suicidal thoughts or behavior with AEDs was observed as early as oneweek after starting drug treatment with AEDs and persisted for the duration oftreatment assessed. Because most trials included in the analysis did not extendbeyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weekscould not be assessed.
The risk ofsuicidal thoughts or behavior was generally consistent among drugs in the dataanalyzed. The finding of increased risk with AEDs of varying mechanisms ofaction and across a range of indications suggests that the risk applies to allAEDs used for any indication. The risk did not vary substantially by age (5-100years) in the clinical trials analyzed. Table 2 shows absolute and relativerisk by indication for all evaluated AEDs.
TABLE 2 Risk by Indication for Antiepileptic Drugs in the PooledAnalysis
Indication
Placebo Patients with Events Per 1000 Patients
Drug Patients with Events Per 1000 Patients
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events Per 1000 Patients
Epilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relativerisk for suicidal thoughts or behavior was higher in clinical trials forepilepsy than in clinical trials for psychiatric or other conditions, but theabsolute risk differences were similar for the epilepsy and psychiatricindications.
Anyoneconsidering prescribing gabapentin or any other AED must balance the risk ofsuicidal thoughts or behavior with the risk of untreated illness. Epilepsy andmany other illnesses for which AEDs are prescribed are themselves associatedwith morbidity and mortality and an increased risk of suicidal thoughts andbehavior. Should suicidal thoughts and behavior emerge during treatment, theprescriber needs to consider whether the emergence of these symptoms in anygiven patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDsincrease the risk of suicidal thoughts and behavior and should be advised ofthe need to be alert for the emergence or worsening of the signs and symptomsof depression, any unusual changes in mood or behavior, or the emergence ofsuicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concernshould be reported immediately to healthcare providers.
5.7 Neuropsychiatric Adverse Reactions (Pediatric Patients 3 to 12 Years of Age)
Gabapentin usein pediatric patients with epilepsy 3-12 years of age is associated with theoccurrence of central nervous system related adverse reactions. The mostsignificant of these can be classified into the following categories: 1)emotional lability (primarily behavioral problems), 2) hostility, includingaggressive behaviors, 3) thought disorder, including concentration problems andchange in school performance, and 4) hyperkinesia (primarily restlessness andhyperactivity). Among the gabapentin-treated patients, most of the reactionswere mild to moderate in intensity.
In controlled clinical epilepsy trials in pediatric patients 3 to 12years of age, the incidence of these adverse reactions was: emotional lability6% (gabapentin-treated patients) vs. 1.3% (placebo-treated patients); hostility5.2% vs. 1.3%; hyperkinesia 4.7% vs. 2.9%; and thought disorder 1.7% vs. 0%.One of these reactions, a report of hostility, was considered serious.Discontinuation of gabapentin treatment occurred in 1.3% of patients reportingemotional lability and hyperkinesia and 0.9% of gabapentin-treated patientsreporting hostility and thought disorder. One placebo-treated patient (0.4%)withdrew due to emotional lability.
5.8 Tumorigenic Potential
In an oral carcinogenicity study, gabapentin increased the incidence ofpancreatic acinar cell tumors in rats [see Nonclinical Toxicology (13.1)].The clinical significance of this finding is unknown. Clinical experienceduring gabapentin’s premarketing development provides no direct means to assessits potential for inducing tumors in humans.
In clinical studies in adjunctive therapy in epilepsy comprising 2085patient-years of exposure in patients >12 years of age, new tumors were reportedin 10 patients (2 breast, 3 brain, 2 lung, 1 adrenal, 1 non-Hodgkin’s lymphoma,1 endometrial carcinoma in situ), and preexisting tumors worsened in 11patients (9 brain, 1 breast, 1 prostate) during or up to 2 years followingdiscontinuation of gabapentin.Without knowledge of the background incidence and recurrence in a similarpopulation not treated with gabapentin, it is impossible to know whether the incidence seen in this cohort isor is not affected by treatment.
5.9 Sudden and Unexplained Death in Patients with Epilepsy
During thecourse of premarketing development of gabapentin, 8 sudden and unexplaineddeaths were recorded among a cohort of 2203 epilepsy patients treated (2103patient-years of exposure) with gabapentin.
Some of these could represent seizure-related deaths in which theseizure was not observed, e.g., at night. This represents an incidence of0.0038 deaths per patient-year. Although this rate exceeds that expected in ahealthy population matched for age and sex, it is within the range of estimatesfor the incidence of sudden unexplained deaths in patients with epilepsy notreceiving gabapentin (ranging from 0.0005 for the general population ofepileptics to 0.003 for a clinical trial population similar to that in thegabapentin program, to 0.005 for patients with refractory epilepsy).Consequently, whether these figures are reassuring or raise further concerndepends on comparability of the populations reported upon to the gabapentincohort and the accuracy of the estimates provided.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections:
· Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.1)]
· Anaphylaxis and Angioedema [see Warnings and Precautions (5.2)]
· Somnolence/Sedation and Dizziness [seeWarnings and Precautions (5.4)]
· Withdrawal Precipitated Seizure, Status Epilepticus [see Warnings and Precautions (5.5)]
· Suicidal Behavior and Ideation [see Warnings and Precautions (5.6)]
· Neuropsychiatric Adverse Reactions (Pediatric Patients 3-12 Years of Age) [see Warnings and Precautions (5.7]
· Sudden and Unexplained Death in Patients with Epilepsy [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Postherpetic Neuralgia
The most common adverse reactions associated with the use of gabapentin in adults, not seen at an equivalent frequency among placebo-treated patients, were dizziness, somnolence, and peripheral edema.
In the 2 controlled trials in postherpetic neuralgia, 16% of the 336 patients who received gabapentin and 9% of the 227 patients who received placebo discontinued treatment because of an adverse reaction. The adverse reactions that most frequently led to withdrawal in gabapentin-treated patients were dizziness, somnolence, and nausea.
Table 3 lists adverse reactions that occurred in at least 1% of gabapentin-treated patients with postherpetic neuralgia participating in placebo-controlled trials and that were numerically more frequent in the gabapentin group than in the placebo group.
TABLE 3. Adverse Reactions in Pooled Placebo-Controlled Trials in Postherpetic Neuralgia
Gabapentin N=336%
Placebo
N=227%
Body as a Whole
Asthenia
6
5
Infection
5
4
Accidental injury
3
1
Digestive System
Diarrhea
6
3
Dry mouth
5
1
Constipation
4
2
Nausea
4
3
Vomiting
3
2
Metabolic and Nutritional Disorders
Peripheral edema
8
2
Weight gain
2
0
Hyperglycemia
1
0
Nervous System
Dizziness
28
8
Somnolence
21
5
Ataxia
3
0
Abnormal thinking
3
0
Abnormal gait
2
0
Incoordination
2
0
Respiratory System
Pharyngitis
1
0
Special Senses
Amblyopiaa
3
1
Conjunctivitis
1
0
Diplopia
1
0
Otitis media
1
0
aReported as blurred vision
Other reactions in more than 1% of patients but equally or more frequent in the placebo group included pain, tremor, neuralgia, back pain, dyspepsia, dyspnea, and flu syndrome.
There were no clinically important differences between men and women in the types and incidence of adverse reactions. Because there were few patients whose race was reported as other than white, there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Epilepsy with Partial Onset Seizures (Adjunctive Therapy)
The most common adverse reactions associated with the use of gabapentin in combination with other antiepileptic drugs in patients >12 years of age, not seen at an equivalent frequency among placebo-treated patients, were somnolence, dizziness, ataxia, fatigue, and nystagmus.
The most common adverse reactions reported with the use of gabapentin in combination with other antiepileptic drugs in pediatric patients 3 to 12 years of age, not seen at an equal frequency among placebo-treated patients, were viral infection, fever, nausea and/or vomiting, somnolence, and hostility [see Warnings and Precautions (5.5)].
Approximately 7% of the 2074 patients >12 years of age and approximately 7% of the 449 pediatric patients 3 to 12 years of age who received gabapentin in premarketing clinical trials discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with withdrawal in patients >12 years of age were somnolence (1.2%), ataxia (0.8%), fatigue (0.6%), nausea and/or vomiting (0.6%), and dizziness (0.6%). The adverse reactions most commonly associated with withdrawal in pediatric patients were emotional lability (1.6%), hostility (1.3%), and hyperkinesia (1.1%).
Table 4 lists adverse reactions that occurred in at least 1% of gabapentin-treated patients >12 years of age with epilepsy participating in placebo-controlled trials and were numerically more common in the gabapentin group. In these studies, either gabapentin or placebo was added to the patient’s current antiepileptic drug therapy.
TABLE 4. Adverse Reactions in Pooled Placebo-Controlled Add-On Trials In Epilepsy Patients >12 years of age
Gabapentina N=543 %
Placeboa
N=378 %
Body as a Whole
Fatigue
11
5
Increased Weight
3
2
Back Pain
2
1
Peripheral Edema
2
1
Cardiovascular
Vasodilatation
1
0
Digestive System
Dyspepsia
2
1
Dry Mouth or Throat
2
1
Constipation
2
1
Dental Abnormalities
2
0
Nervous System
Somnolence
19
9
Dizziness
17
7
Ataxia
13
6
Nystagmus
8
4
Tremor
7
3
Dysarthria
2
1
Amnesia
2
0
Depression
2
1
Abnormal thinking
2
1
Abnormal coordination
1
0
Respiratory System
Pharyngitis
3
2
Coughing
2
1
Skin and Appendages
Abrasion
1
0
Urogenital System
Impotence
2
1
Special Senses
Diplopia
6
2
Amblyopiab
4
1
a Plus background antiepileptic drug therapy
b Amblyopia was often described as blurred vision.
Among the adverse reactions occurring at an incidence of at least 10% in gabapentin-treated patients, somnolence and ataxia appeared to exhibit a positive dose-response relationship.
The overall incidence of adverse reactions and the types of adverse reactions seen were similar among men and women treated with gabapentin. The incidence of adverse reactions increased slightly with increasing age in patients treated with either gabapentin or placebo. Because only 3% of patients (28/921) in placebo-controlled studies were identified as nonwhite (black or other), there are insufficient data to support a statement regarding the distribution of adverse reactions by race.
Table 5 lists adverse reactions that occurred in at least 2% of gabapentin-treated patients, age 3 to 12 years of age with epilepsy participating in placebo-controlled trials, and which were numerically more common in the gabapentin group.
TABLE 5. Adverse Reactions in a Placebo-Controlled Add-On Trial in Pediatric Epilepsy Patients Age 3 to 12 Years
Gabapentina N=119 %
Placeboa
N=128 %
Body as a Whole
Viral Infection
11
3
Fever
10
3
Increased Weight
3
1
Fatigue
3
2
Digestive System
Nausea and/or Vomiting
8
7
Nervous System
Somnolence
8
5
Hostility
8
2
Emotional Lability
4
2
Dizziness
3
2
Hyperkinesia
3
1
Respiratory System
Bronchitis
3
1
Respiratory Infection
3
1
a Plus background antiepileptic drug therapy
Other reactions in more than 2% of pediatric patients 3 to 12 years of age but equally or more frequent in the placebo group included: pharyngitis, upper respiratory infection, headache, rhinitis, convulsions, diarrhea, anorexia, coughing, and otitis media.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of gabapentin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary disorders: jaundice Investigations: elevated creatine kinase, elevated liver function tests Metabolism and nutrition disorders: hyponatremia
Musculoskeletal and connective tissue disorder: rhabdomyolysis
Nervous system disorders: movement disorder Reproductive system and breast disorders: breast enlargement, changes in libido, ejaculation disorders and anorgasmia
Skin and subcutaneous tissue disorders: angioedema [see Warnings and Precautions (5.2)], erythema multiforme, Stevens-Johnson syndrome.
Adverse reactions following the abrupt discontinuation of gabapentin have also been reported. The most frequently reported reactions were anxiety, insomnia, nausea, pain, and sweating.
-
7 DRUG INTERACTIONS
7.1 Other Antiepileptic Drugs
Gabapentin is not appreciably metabolized nor does it interfere with the metabolism of commonly coadministered antiepileptic drugs [see Clinical Pharmacology (12.3)].
7.2 Opioids
Hydrocodone
Coadministration of gabapentin with hydrocodone decreases hydrocodone exposure [see Clinical Pharmacology (12.3)]. The potential for alteration in hydrocodone exposure and effect should be considered when gabapentin is started or discontinued in a patient taking hydrocodone.
Morphine
When gabapentin is administered with morphine, patients should be observed for signs of central nervous system (CNS) depression, such as somnolence, sedation and respiratory depression [see Clinical Pharmacology (12.3)].
7.3 Maalox® (aluminum hydroxide, magnesium hydroxide)
The mean bioavailability of gabapentin was reduced by about 20% with concomitant use of an antacid (Maalox®) containing magnesium and aluminum hydroxides. It is recommended that gabapentin be taken at least 2 hours following Maalox administration [see Clinical Pharmacology (12.3)].
7.4 Drug/Laboratory Test Interactions
Because false positive readings were reported withthe Ames N-Multistix SG® dipstick test for urinary protein whengabapentin was added to other antiepileptic drugs, the more specificsulfosalicylic acid precipitation procedure is recommended to determine thepresence of urine protein.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
PregnancyCategory C: There are noadequate and well-controlled studies in pregnant women. In nonclinical studiesin mice, rats, and rabbits, gabapentin was developmentally toxic whenadministered to pregnant animals at doses similar to or lower than those usedclinically. Gabapentin should be used during pregnancy only if the potentialbenefit justifies the potential risk to the fetus.
When pregnantmice received oral doses of gabapentin (500, 1000, or 3000 mg/kg/day) duringthe period of organogenesis, embryo-fetal toxicity (increased incidences ofskeletal variations) was observed at the two highest doses. The no-effect dosefor embryo-fetal developmental toxicity in mice was 500 mg/kg/day orapproximately ½ of the maximum recommended human dose (MRHD) of 3600 mg/kg on abody surface area (mg/m2) basis.
In studies in which rats received oral doses of gabapentin (500 to 2000 mg/Kg/day) during pregnancy,adverse effect on offspring development (increased incidences of hydroureterand/or hydronephrosis) were observed at all doses. The lowest effect dose fordevelopmental toxicity in rats is approximately equal to the MRHD on a mg/m2basis.
When pregnantrabbits were treated with gabapentin during the period of organogenesis, anincrease in embryo-fetal mortality was observed at all doses tested (60, 300,or 1500 mg/kg). The lowest effect dose for embryo-fetal developmental toxicityin rabbits is less than the MRHD on a mg/m2 basis.
In a publishedstudy, gabapentin (400 mg/kg/day) was administered by intraperitoneal injectionto neonatal mice during the first postnatal week, a period of synaptogenesis inrodents (corresponding to the last trimester of pregnancy in humans).Gabapentin caused a marked decrease in neuronal synapse formation in brains ofintact mice and abnormal neuronal synapse formation in a mouse model ofsynaptic repair. Gabapentin has been shown in vitro to interfere withactivity of the α2δ subunit of voltage-activated calcium channels, a receptorinvolved in neuronal synaptogenesis. The clinical significance of thesefindings is unknown.
To provide information regarding the effects of in utero exposureto gabapentin, physicians are advised to recommend that pregnant patientstaking gabapentin enroll in the North American Antiepileptic Drug (NAAED)Pregnancy Registry. This can be done by calling the toll free number1-888-233-2334, and must be done by patients themselves. Information on theregistry can also be found at the website http://www.aedpregnancyregistry.org/.
8.3 Nursing Mothers
Gabapentin is secreted into human milk following oral administration. A nursed infant could be exposed to a maximum dose of approximately 1 mg/kg/day of gabapentin. Because the effect on the nursing infant is unknown, gabapentin should be used in women who are nursing only if the benefits clearly outweigh the risks.
8.4 Pediatric Use
Safety and effectiveness of gabapentin in the management of postherpetic neuralgia in pediatric patients have not been established.
Effectiveness as adjunctive therapy in the treatment of partial seizures in pediatric patients below the age of 3 years has not been established [see Clinical Studies (14.2)].
8.5 Geriatric Use
The total number of patients treated with gabapentin in controlled clinical trials in patients with postherpetic neuralgia was 336, of which 102 (30%) were 65 to 74 years of age, and 168 (50%) were 75 years of age and older. There was a larger treatment effect in patients 75 years of age and older compared with younger patients who received the same dosage. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥ 75 years may be a consequence of increased gabapentin exposure for a given dose that results from an age-related decrease in renal function. However, other factors cannot be excluded. The types and incidence of adverse reactions were similar across age groups except for peripheral edema and ataxia, which tended to increase in incidence with age.
Clinical studies of gabapentin in epilepsy did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and dose should be adjusted based on creatinine clearance values in these patients [see Dosage and Administration (2.4), Adverse Reactions (6), and Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Dosage adjustment in adult patients with compromised renal function is necessary [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. Pediatric patients with renal insufficiency have not been studied.
Dosage adjustment in patients undergoing hemodialysis is necessary [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Gabapentin does not exhibit affinity for benzodiazepine, opiate (mu, delta or kappa), or cannabinoid 1 receptor sites. A small number of postmarketing cases report gabapentin misuse and abuse. These individuals were taking higher than recommended doses of gabapentin for unapproved uses. Most of the individuals described in these reports had a history of poly-substance abuse or used gabapentin to relieve symptoms of withdrawal from other substances. When prescribing gabapentin carefully evaluate patients for a history of drug abuse and observe them for signs and symptoms of gabapentin misuse or abuse (e.g. development of tolerance, self-dose escalation, and drug-seeking behavior).
9.3 Dependence
There are rare postmarketing reports of individuals experiencing withdrawal symptoms shortly after discontinuing higher than recommended doses of gabapentin used to treat illnesses for which the drug is not approved. Such symptoms included agitation, disorientation and confusion after suddenly discontinuing gabapentin that resolved after restarting gabapentin. Most of these individuals had a history of poly-substance abuse or used gabapentin to relieve symptoms of withdrawal from other substances. The dependence and abuse potential of gabapentin has not been evaluated in human studies.
-
10 OVERDOSAGE
A lethal dose of gabapentin was not identified in mice and rats receiving single oral doses as high as 8000 mg/kg. Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, sedation, hypoactivity, or excitation.
Acute oral overdoses of gabapentin up to 49 grams have been reported. In these cases, double vision, slurred speech, drowsiness, lethargy, and diarrhea were observed. All patients recovered with supportive care. Coma, resolving with dialysis, has been reported in patients with chronic renal failure who were treated with gabapentin.
Gabapentin can be removed by hemodialysis. Although hemodialysis has not been performed in the few overdose cases reported, it may be indicated by the patients clinical state or in patients with significant renal impairment.
If overexposure occurs, call your poison control center at 1-800-222-1222.
-
11 DESCRIPTION
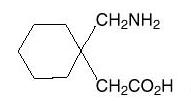
The active ingredient in gabapentin capsules isgabapentin, which has the chemical name 1-(aminomethyl) cyclohexaneacetic acid.The molecular formula of gabapentin is C9H17NO2and the molecular weight is 171.24. The structural formula of gabapentin is:
Gabapentin is awhite to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. Itis freely soluble in water and both basic and acidic aqueous solutions. The logof the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is–1.25.
Each gabapentin capsule contains 100 mg, 300 mg, or 400 mg of gabapentinand the following inactive ingredients: anhydrous lactose, cornstarch, andtalc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate, andtitanium dioxide. The 300 mg capsule shell contains gelatin, sodium laurylsulfate, titanium dioxide, and yellow iron oxide. The 400 mg capsule shellcontains gelatin, sodium lauryl sulfate, red iron oxide, titanium dioxide, andyellow iron oxide. The imprinting ink contains shellac, dehydrated alcohol,isopropyl alcohol, butyl alcohol, propyl glycol, strong ammonia solution, andtitanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanisms by which gabapentin producesits analgesic and antiepileptic actions are unknown. Gabapentin is structurallyrelated to the neurotransmitter gamma-aminobutyric acid (GABA) but has noeffect on GABA binding, uptake, or degradation. In vitro studies haveshown that gabapentin binds with high-affinity to the α2δ subunit ofvoltage-activated calcium channels; however, the relationship of this bindingto the therapeutic effects of gabapentin is unknown.
12.3 Pharmacokinetics
All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans.
Oral Bioavailability
Gabapentin bioavailability is not dose proportional; i.e., as dose is increased, bioavailability decreases. Bioavailability of gabapentin is approximately 60%, 47%, 34%, 33%, and 27% following 900, 1200, 2400, 3600, and 4800 mg/day given in 3 divided doses, respectively. Food has only a slight effect on the rate and extent of absorption of gabapentin (14% increase in AUC and Cmax).
Distribution
Less than 3% of gabapentin circulates bound to plasma protein. The apparent volume of distribution of gabapentin after 150 mg intravenous administration is 58±6 L (mean ±SD). In patients with epilepsy, steady-state predose (Cmin) concentrations of gabapentin in cerebrospinal fluid were approximately 20% of the corresponding plasma concentrations.
Elimination
Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans.
Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In elderly patients, and in patients with impaired renal function, gabapentin plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis.
Specific Populations
Age
The effect of age was studied in subjects 20-80 years of age. Apparent oral clearance (CL/F) of gabapentin decreased as age increased, from about 225 mL/min in those under 30 years of age to about 125 mL/min in those over 70 years of age. Renal clearance (CLr) and CLr adjusted for body surface area also declined with age; however, the decline in the renal clearance of gabapentin with age can largely be explained by the decline in renal function. [see Dosage and Administration (2.4) and Use in Specific Populations (8.5)].
Gender
Although no formal study has been conducted to compare the pharmacokinetics of gabapentin in men and women, it appears that the pharmacokinetic parameters for males and females are similar and there are no significant gender differences.
Race
Pharmacokinetic differences due to race have not been studied. Because gabapentin is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.
Pediatric
Gabapentin pharmacokinetics were determined in 48 pediatric subjects between the ages of 1 month and 12 years following a dose of approximately 10 mg/kg. Peak plasma concentrations were similar across the entire age group and occurred 2 to 3 hours postdose. In general, pediatric subjects between 1 month and <5 years of age achieved approximately 30% lower exposure (AUC) than that observed in those 5 years of age and older. Accordingly, oral clearance normalized per body weight was higher in the younger children. Apparent oral clearance of gabapentin was directly proportional to creatinine clearance. Gabapentin elimination half-life averaged 4.7 hours and was similar across the age groups studied.
A population pharmacokinetic analysis was performed in 253 pediatric subjects between 1 month and 13 years of age. Patients received 10 to 65 mg/kg/day given three times a day. Apparent oral clearance (CL/F) was directly proportional to creatinine clearance and this relationship was similar following a single dose and at steady state. Higher oral clearance values were observed in children <5 years of age compared to those observed in children 5 years of age and older, when normalized per body weight. The clearance was highly variable in infants <1 year of age. The normalized CL/F values observed in pediatric patients 5 years of age and older were consistent with values observed in adults after a single dose. The oral volume of distribution normalized per body weight was constant across the age range.
These pharmacokinetic data indicate that the effective daily dose in pediatric patients with epilepsy ages 3 and 4 years should be 40 mg/kg/day to achieve average plasma concentrations similar to those achieved in patients 5 years of age and older receiving gabapentin at 30 mg/kg/day [see Dosage and Administration (2.1)].
Adult Patients with Renal Impairment
Subjects (N=60) with renal impairment (mean creatinine clearance ranging from 13-114 mL/min) were administered single 400 mg oral doses of gabapentin. The mean gabapentin half-life ranged from about 6.5 hours (patients with creatinine clearance >60 mL/min) to 52 hours (creatinine clearance <30 mL/min) and gabapentin renal clearance from about 90 mL/min (>60 mL/min group) to about 10 mL/min (<30 mL/min). Mean plasma clearance (CL/F) decreased from approximately 190 mL/min to 20 mL/min [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)]. Pediatric patients with renal insufficiency have not been studied.
Hemodialysis
In a study in anuric adult subjects (N=11), the apparent elimination half-life of gabapentin on nondialysis days was about 132 hours; during dialysis the apparent half-life of gabapentin was reduced to 3.8 hours. Hemodialysis thus has a significant effect on gabapentin elimination in anuric subjects [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
Hepatic Disease
Because gabapentin is not metabolized, no study was performed in patients with hepatic impairment.
Drug Interactions
In Vitro Studies
In vitro studies were conducted to investigate the potential of gabapentin to inhibit the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) that mediate drug and xenobiotic metabolism using isoform selective marker substrates and human liver microsomal preparations. Only at the highest concentration tested (171 mcg/mL; 1 mM) was a slight degree of inhibition (14%-30%) of isoform CYP2A6 observed. No inhibition of any of the other isoforms tested was observed at gabapentin concentrations up to 171 mcg/mL (approximately 15 times the Cmax at 3600 mg/day).
· In Vivo Studies
The drug interaction data described in this section were obtained from studies involving healthy adults and adult patients with epilepsy.
Phenytoin
In a single (400 mg) and multiple dose (400 mg three times a day) study of gabapentin in epileptic patients (N=8) maintained on phenytoin monotherapy for at least 2 months, gabapentin had no effect on the steady-state trough plasma concentrations of phenytoin and phenytoin had no effect on gabapentin pharmacokinetics.
Carbamazepine
Steady-state trough plasma carbamazepine and carbamazepine 10, 11 epoxide concentrations were not affected by concomitant gabapentin (400 mg three times a day; N=12) administration. Likewise, gabapentin pharmacokinetics were unaltered by carbamazepine administration.
Valproic Acid
The mean steady-state trough serum valproic acid concentrations prior to and during concomitant gabapentin administration (400 mg three times a day; N=17) were not different and neither were gabapentin pharmacokinetic parameters affected by valproic acid.
Phenobarbital
Estimates of steady-state pharmacokinetic parameters for phenobarbital or gabapentin (300 mg three times a day; N=12) are identical whether the drugs are administered alone or together.
Naproxen
Coadministration (N=18) of naproxen sodium capsules (250 mg) with gabapentin (125 mg) appears to increase the amount of gabapentin absorbed by 12% to 15%. Gabapentin had no effect on naproxen pharmacokinetic parameters. These doses are lower than the therapeutic doses for both drugs. The magnitude of interaction within the recommended dose ranges of either drug is not known.
Hydrocodone
Coadministration of gabapentin (125 to 500 mg; N=48) decreases hydrocodone (10 mg; N=50) Cmax and AUC values in a dose-dependent manner relative to administration of hydrocodone alone; Cmax and AUC values are 3% to 4% lower, respectively, after administration of 125 mg gabapentin and 21% to 22% lower, respectively, after administration of 500 mg gabapentin. The mechanism for this interaction is unknown. Hydrocodone increases gabapentin AUC values by 14%. The magnitude of interaction at other doses is not known.
Morphine
A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg gabapentin capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Morphine pharmacokinetic parameter values were not affected by administration of gabapentin 2 hours after morphine. The magnitude of interaction at other doses is not known.
Cimetidine
In the presence of cimetidine at 300 mg QID (N=12), the mean apparent oral clearance of gabapentin fell by 14% and creatinine clearance fell by 10%. Thus, cimetidine appeared to alter the renal excretion of both gabapentin and creatinine, an endogenous marker of renal function. This small decrease in excretion of gabapentin by cimetidine is not expected to be of clinical importance. The effect of gabapentin on cimetidine was not evaluated.
Oral Contraceptive
Based on AUC and half-life, multiple-dose pharmacokinetic profiles of norethindrone and ethinyl estradiol following administration of tablets containing 2.5 mg of norethindrone acetate and 50 mcg of ethinyl estradiol were similar with and without coadministration of gabapentin (400 mg three times a day; N=13). The Cmax of norethindrone was 13% higher when it was coadministered with gabapentin; this interaction is not expected to be of clinical importance.
Antacid (Maalox®) (aluminum hydroxide, magnesium hydroxide)
Antacid (Maalox®) containing magnesium and aluminum hydroxides reduced the mean bioavailability of gabapentin (N=16) by about 20%. This decrease in bioavailability was about 10% when gabapentin was administered 2 hours after Maalox.
Probenecid
Probenecid is a blocker of renal tubular secretion. Gabapentin pharmacokinetic parameters without and with probenecid were comparable. This indicates that gabapentin does not undergo renal tubular secretion by the pathway that is blocked by probenecid.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
Gabapentin wasadministered orally to mice and rats in 2-year carcinogenicity studies. Noevidence of drug-related carcinogenicity was observed in mice treated at dosesup to 2000 mg/kg/day. At 2000 mg/kg, the plasma gabapentin exposure (AUC) inmice is approximately 2 times that in humans at the MRHD of 3600 mg/day. Inrats, increases in the incidence of pancreatic acinar cell adenoma andcarcinoma were found in male rats receiving the highest dose (2000 mg/kg), butnot at doses of 250 or 1000 mg/kg/day. At 1000 mg/kg, the plasma gabapentinexposure (AUC) in rats is approximately 5 times that in humans at the MRHD.
Studiesdesigned to investigate the mechanism of gabapentin-induced pancreaticcarcinogenesis in rats indicate that gabapentin stimulates DNA synthesis in ratpancreatic acinar cells in vitro and, thus, may be acting as a tumorpromoter by enhancing mitogenic activity. It is not known whether gabapentinhas the ability to increase cell proliferation in other cell types or in otherspecies, including humans.
Gabapentin didnot demonstrate mutagenic or genotoxic potential in three in vitro andfour in vivo assays. It was negative in the Ames test and the invitro HGPRT forward mutation assay in Chinese hamster lung cells; it didnot produce significant increases in chromosomal aberrations in the in vitroChinese hamster lung cell assay; it was negative in the in vivo chromosomalaberration assay and in the in vivo micronucleus test in Chinese hamsterbone marrow; it was negative in the in vivo mouse micronucleus assay;and it did not induce unscheduled DNA synthesis in hepatocytes from rats givengabapentin.
No adverse effects on fertility or reproduction were observed in rats atdoses up to 2000 mg/kg. At 2000 mg/kg, the plasma gabapentin exposure (AUC) inrats is approximately 8 times that in humans at the MRHD.
-
14 CLINICAL STUDIES
14.1 Postherpetic Neuralgia
Gabapentin wasevaluated for the management of postherpetic neuralgia (PHN) in two randomized,double-blind, placebo-controlled, multicenter studies. The intent-to-treat(ITT) population consisted of a total of 563 patients with pain for more than 3months after healing of the herpes zoster skin rash (Table 6).
TABLE 6. Controlled PHN Studies: Duration, Dosages, and Number ofPatients
Study
Study Duration
Gabapentin (mg/day)a Target Dose
Patients Receiving Gabapentin
Patients Receiving Placebo
1
8 weeks
3600
113
116
2
7 weeks
1800, 2400
223
111
Total
336
227
aGiven in 3 divided doses (TID)
Each studyincluded a 7- or 8-week double-blind phase (3 or 4 weeks of titration and 4weeks of fixed dose). Patients initiated treatment with titration to a maximumof 900 mg/day gabapentin over 3 days. Dosages were then to be titrated in 600to 1200 mg/day increments at 3- to 7-day intervals to the target dose over 3 to4 weeks. Patients recorded their pain in a daily diary using an 11-pointnumeric pain rating scale ranging from 0 (no pain) to 10 (worst possible pain).A mean pain score during baseline of at least 4 was required for randomization.Analyses were conducted using the ITT population (all randomized patients whoreceived at least one dose of study medication).
Both studiesdemonstrated efficacy compared to placebo at all doses tested.
The reduction in weekly mean pain scores was seen by Week 1 in bothstudies, and were maintained to the end of treatment. Comparable treatmenteffects were observed in all active treatment arms.Pharmacokinetic/pharmacodynamic modeling provided confirmatory evidence ofefficacy across all doses. Figures 1 and 2 show pain intensity scores over timefor Studies 1 and 2.
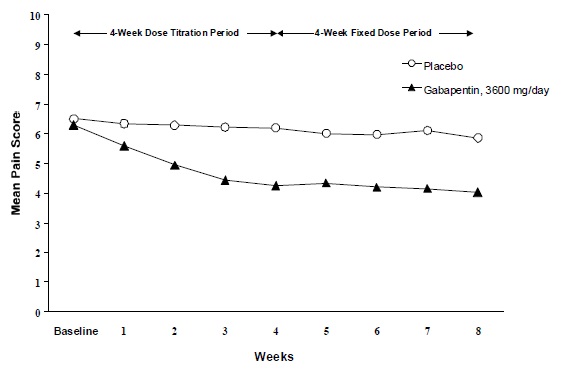
Figure 1. Weekly Mean Pain Scores (Observed Casesin ITT Population): Study 1
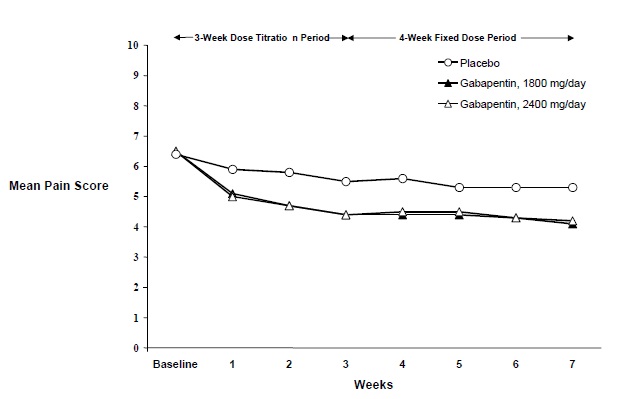
Figure 2. Weekly Mean Pain Scores (Observed Casesin ITT Population): Study 2
The proportion of responders (those patientsreporting at least 50% improvement in endpoint pain score compared withbaseline) was calculated for each study (Figure 3).
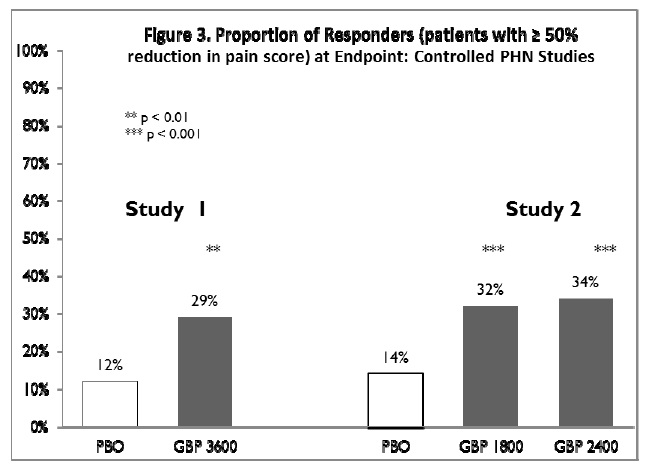
Figure 3. Proportion of Responders (patients with ≥50%reduction in pain score) at Endpoint: Controlled PHN Studies
14.2 Epilepsy for Partial Onset Seizures (Adjunctive Therapy)
Theeffectiveness of gabapentin as adjunctive therapy (added to other antiepilepticdrugs) was established in multicenter placebo-controlled, double-blind,parallel-group clinical trials in adult and pediatric patients (3 years andolder) with refractory partial seizures.
Evidence ofeffectiveness was obtained in three trials conducted in 705 patients (age 12years and above) and one trial conducted in 247 pediatric patients (3 to 12years of age). The patients enrolled had a history of at least 4 partialseizures per month in spite of receiving one or more antiepileptic drugs attherapeutic levels and were observed on their established antiepileptic drugregimen during a 12-week baseline period (6 weeks in the study of pediatricpatients). In patients continuing to have at least 2 (or 4 in some studies)seizures per month, gabapentin or placebo was then added on to the existingtherapy during a 12-week treatment period. Effectiveness was assessed primarilyon the basis of the percent of patients with a 50% or greater reduction inseizure frequency from baseline to treatment (the “responder rate”) and aderived measure called response ratio, a measure of change defined as (T -B)/(T + B), in which B is the patient’s baseline seizure frequency and T is thepatient’s seizure frequency during treatment. Response ratio is distributedwithin the range -1 to +1. A zero value indicates no change while completeelimination of seizures would give a value of -1; increased seizure rates wouldgive positive values. A response ratio of -0.33 corresponds to a 50% reductionin seizure frequency. The results given below are for all partial seizures inthe intent-to-treat (all patients who received any doses of treatment)population in each study, unless otherwise indicated.
One studycompared gabapentin 1200 mg/day, in three divided doses with placebo. Responderrate was 23% (14/61) in the gabapentin group and 9% (6/66) in the placebogroup; the difference between groups was statistically significant. Responseratio was also better in the gabapentin group (-0.199) than in the placebogroup (-0.044), a difference that also achieved statistical significance.
A second studycompared primarily gabapentin 1200 mg/day, in three divided doses (N=101), withplacebo (N=98). Additional smaller gabapentin dosage groups (600 mg/day, N=53;1800 mg/day, N=54) were also studied for information regarding dose response.Responder rate was higher in the gabapentin 1200 mg/day group (16%) than in theplacebo group (8%), but the difference was not statistically significant. Theresponder rate at 600 mg (17%) was also not significantly higher than in theplacebo, but the responder rate in the 1800 mg group (26%) was statisticallysignificantly superior to the placebo rate. Response ratio was better in thegabapentin 1200 mg/day group (-0.103) than in the placebo group (-0.022); butthis difference was also not statistically significant (p = 0.224). A betterresponse was seen in the gabapentin 600 mg/day group (-0.105) and 1800 mg/daygroup (-0.222) than in the 1200 mg/day group, with the 1800 mg/day groupachieving statistical significance compared to the placebo group.
A third studycompared gabapentin 900 mg/day, in three divided doses (N=111), and placebo(N=109). An additional gabapentin 1200 mg/day dosage group (N=52) provideddose-response data. A statistically significant difference in responder ratewas seen in the gabapentin 900 mg/day group (22%) compared to that in theplacebo group (10%). Response ratio was also statistically significantlysuperior in the gabapentin 900 mg/day group (-0.119) compared to that in theplacebo group (-0.027), as was response ratio in 1200 mg/day gabapentin(-0.184) compared to placebo.
Analyses werealso performed in each study to examine the effect of gabapentin on preventingsecondarily generalized tonic-clonic seizures. Patients who experienced asecondarily generalized tonic-clonic seizure in either the baseline or in thetreatment period in all three placebo-controlled studies were included in theseanalyses. There were several response ratio comparisons that showed astatistically significant advantage for gabapentin compared to placebo andfavorable trends for almost all comparisons.
Analysis ofresponder rate using combined data from all three studies and all doses (N=162,gabapentin; N=89, placebo) also showed a significant advantage for gabapentinover placebo in reducing the frequency of secondarily generalized tonic-clonicseizures.
In two of the three controlled studies, more than one dose of gabapentinwas used. Within each study, the results did not show a consistently increasedresponse to dose. However, looking across studies, a trend toward increasingefficacy with increasing dose is evident (see Figure 4).
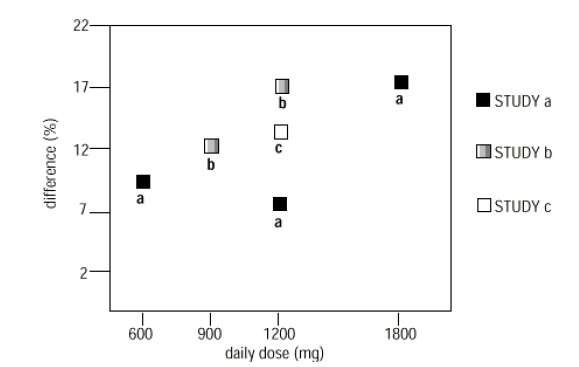
Figure 4.Responder Rate in Patients Receiving gabapentin Expressed as a Difference fromPlacebo by Dose and Study: Adjunctive Therapy Studies in Patients ≥ 12 Years ofAge with Partial Seizures
In the figure,treatment effect magnitude, measured on the Y axis in terms of the differencein the proportion of gabapentin and placebo-assigned patients attaining a 50%or greater reduction in seizure frequency from baseline, is plotted against thedaily dose of gabapentin administered (X axis).
Although noformal analysis by gender has been performed, estimates of response (ResponseRatio) derived from clinical trials (398 men, 307 women) indicate no important genderdifferences exist. There was no consistent pattern indicating that age had anyeffect on the response to gabapentin. There were insufficient numbers ofpatients of races other than Caucasian to permit a comparison of efficacy amongracial groups.
A fourth studyin pediatric patients age 3 to 12 years compared 25 – 35 mg/kg/day gabapentin(N=118) with placebo (N=127). For all partial seizures in the intent-to-treatpopulation, the response ratio was statistically significantly better for thegabapentin group (-0.146) than for the placebo group (-0.079). For the samepopulation, the responder rate for gabapentin (21%) was not significantlydifferent from placebo (18%).
A study in pediatric patients age 1 month to 3 years compared 40mg/kg/day gabapentin (N=38) with placebo (N=38) in patients who were receivingat least one marketed antiepileptic drug and had at least one partial seizureduring the screening period (within 2 weeks prior to baseline). Patients had upto 48 hours of baseline and up to 72 hours of double-blind video EEG monitoringto record and count the occurrence of seizures. There were no statisticallysignificant differences between treatments in either the response ratio orresponder rate.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Gabapentincapsules USP is supplied as follows:
100 mgcapsules:
White hardgelatin capsules imprinted “216” on body with blue ink, available in:
Bottles of 100:NDC: 67877-222-01
Bottles of 500:NDC: 67877-222-05
Bottles of1000: NDC: 67877-222-10
NDC 69189-0683-1 single dose pack with 1 capsule as repackaged by Avera McKennan Hospital
Storage
Store at 20° to 25°C (68° to 77°F); [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity
Prior to initiation of treatment with gabapentin, instruct patients that a rash or other signs or symptoms of hypersensitivity (such as fever or lymphadenopathy) may herald a serious medical event and that the patient should report any such occurrence to a physician immediately [see Warnings and Precautions (5.1)] .
Anaphylaxis and Angioedema
Advise patients to discontinue gabapentin and seek medical care if they develop signs or symptoms of anaphylaxis or angioedema [see Warnings and Precautions (5.2)].
Dizziness and Somnolence and Effects on Driving and Operating Heavy Machinery
Advise patients that gabapentin may cause dizziness, somnolence, and other symptoms and signs of CNS depression. Other drugs with sedative properties may increase these symptoms. Accordingly, although patients’ ability to determine their level of impairment can be unreliable, advise them neither to drive a car nor to operate other complex machinery until they have gained sufficient experience on gabapentin to gauge whether or not it affects their mental and/or motor performance adversely. Inform patients that it is not known how long this effect lasts [see Warnings and Precautions (5.3) and Warnings and Precautions (5.4)].
Suicidal Thinking and Behavior
Counsel the patient, their caregivers, and families that AEDs, including gabapentin, may increase the risk of suicidal thoughts and behavior. Advise patients of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients to report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.6)].
Use in Pregnancy
Instruct patients to notify their physician if they become pregnant or intend to become pregnant during therapy, and to notify their physician if they are breast feeding or intend to breast feed during therapy [see Use in Specific Populations (8.1) and (8.3)].
Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 [see Use in Specific Populations (8.1)].
This product’s label may have been updated. For full prescribing information, please visit www.fda.report.
Manufactured by:
Alkem Laboratories Limited
ALKEM HOUSE, Lower Parel,
Mumbai – 400 013, INDIA
Distributed by:
Ascend Laboratories, LCC
Montvale, NJ 07645
PT 1702-03 -
Medication Guide
Gabapentin Capsules USP
(GA ba PEN tin)
Read the Medication Guide before you start taking gabapentin and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about gabapentin?
Do not stop taking gabapentin without first talking to your healthcare provider.
Stopping gabapentin suddenly can cause serious problems.
Gabapentin can cause serious side effects including:
1. Suicidal Thoughts. Like other antiepileptic drugs, gabapentin may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
· thoughts about suicide or dying
· attempts to commit suicide
· new or worse depression
· new or worse anxiety
· feeling agitated or restless
· panic attacks
· trouble sleeping (insomnia)
· new or worse irritability
· acting aggressive, being angry, or violent
· acting on dangerous impulses
· an extreme increase in activity and talking (mania)
· other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
· Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
· Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking gabapentin without first talking to a healthcare provider.
· Stopping gabapentin suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
· Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
2. Changes in behavior and thinking -Using gabapentin in children 3 to 12 years of age can cause emotional changes, aggressive behavior, problems with concentration, restlessness, changes in school performance, and hyperactivity.
3. Gabapentin may cause a serious or life-threatening allergic reactions that may affect your skin or other parts of your body such as your liver or blood cells. This may cause you to be hospitalized or to stop gabapentin. You may or may not have a rash with an allergic reaction caused by gabapentin. Call a healthcare provider right away if you have any of the following symptoms:
· skin rash
· hives
· difficulty breathing
· fever
· swollen glands that do not go away
· swelling of your face, lips, throat, or tongue
· yellowing of your skin or of the whites of the eyes
· unusual bruising or bleeding
· severe fatigue or weakness
· unexpected muscle pain
· frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking gabapentin.
What is gabapentin?
Gabapentin is a prescription medicine used to treat:
· Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults.
· Partial seizures when taken together with other medicines in adults and children 3 years of age and older with seizures.
Who should not take gabapentin?
Do not take gabapentin if you are allergic to gabapentin or any of the other ingredients in gabapentin. See the end of this Medication Guide for a complete list of ingredients in gabapentin.
What should I tell my healthcare provider before taking gabapentin?
Before taking gabapentin, tell your healthcare provider if you:
· have or have had kidney problems or are on hemodialysis
· have or have had depression, mood problems, or suicidal thoughts or behavior
· have diabetes
· are pregnant or plan to become pregnant. It is not known if gabapentin can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking gabapentin. You and your healthcare provider will decide if you should take gabapentin while you are pregnant.
o If you become pregnant while taking gabapentin, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
· are breast-feeding or plan to breast-feed. Gabapentin can pass into breast milk. You and your healthcare provider should decide how you will feed your baby while you take gabapentin.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking gabapentin with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take gabapentin?
· Take gabapentin exactly as prescribed. Your healthcare provider will tell you how much gabapentin to take.
o Do not change your dose of gabapentin without talking to your healthcare provider.
o Take gabapentin capsules with water.
If you take too much gabapentin, call your healthcare provider or your local Poison Control Center right away at 1-800-222-1222.
What should I avoid while taking gabapentin?
· Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking gabapentin without first talking with your healthcare provider. Taking gabapentin with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
· Do not drive, operate heavy machinery, or do other dangerous activities until you know how gabapentin affects you. Gabapentin can slow your thinking and motor skills.
What are the possible side effects of gabapentin?
Gabapentin may cause serious side effects including:
See “What is the most important information I should know about gabapentin?”
· problems driving while using gabapentin. See “What I should avoid while taking gabapentin?”
· sleepiness and dizziness, which could increase the occurrence of accidental injury, including falls
· The most common side effects of gabapentin include:
· lack of coordination
· feeling tired
· viral infection
· fever
· feeling drowsy
· jerky movements
· nausea and vomiting
· difficulty with coordination
· difficulty with speaking
· double vision
· tremor
· unusual eye movement
· swelling, usually of legs and feet
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of gabapentin. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store gabapentin?
· Store gabapentin Capsules between 20° to 25°C (68° to 77°F); [see USP Controlled Room Temperature].
Keep gabapentin and all medicines out of the reach of children.
General information about the safe and effective use of gabapentin
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use gabapentin for a condition for which it was not prescribed. Do not give gabapentin to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about gabapentin. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about gabapentin that was written for healthcare professionals.
What are the ingredients in gabapentin?
Active ingredient: gabapentin
Inactive ingredients in the capsules: anhydrous lactose, cornstarch, and talc. The 100-mg capsule shell also contains: gelatin, sodium lauryl sulfate, and titanium dioxide.
The 300-mg capsule shell also contains: gelatin, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
The 400-mg capsule shell also contains: gelatin, sodium lauryl sulfate, red iron oxide, titanium dioxide, and yellow iron oxide. The imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propyl glycol, strong ammonia solution, and titanium dioxide.
Manufactured by:
Alkem Laboratories Limited
ALKEM HOUSE, Lower Parel,
Mumbai – 400 013, INDIA
Distributed by:
Ascend Laboratories, LCC.
Montvale, NJ 07645
Revised October 2015
PT 1702-03 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GABAPENTIN
gabapentin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69189-0683(NDC:67877-222) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GABAPENTIN (UNII: 6CW7F3G59X) (GABAPENTIN - UNII:6CW7F3G59X) GABAPENTIN 100 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) TALC (UNII: 7SEV7J4R1U) GELATIN (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (White) Score no score Shape CAPSULE (Capsule) Size 14mm Flavor Imprint Code 216 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69189-0683-1 1 in 1 DOSE PACK; Type 0: Not a Combination Product 08/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090858 08/01/2016 Labeler - Avera McKennan Hospital (068647668) Establishment Name Address ID/FEI Business Operations Avera McKennan Hospital 068647668 relabel(69189-0683) , repack(69189-0683)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
