Carprodyl by Ceva Sante Animale / Intas Pharmaceuticals Limited / PROCOS SpA Carprodyl® Injection
Carprodyl by
Drug Labeling and Warnings
Carprodyl by is a Animal medication manufactured, distributed, or labeled by Ceva Sante Animale, Intas Pharmaceuticals Limited , PROCOS SpA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARPRODYL- carprofen injection, solution
Ceva Sante Animale
----------
Carprodyl® Injection
Sterile Injection Solution
50 mg/mL
Non-steroidal anti-inflammatory drug
For subcutaneous use in dogs only
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION:
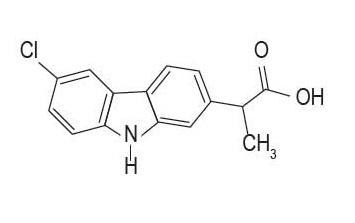
Carprodyl® Injection is a sterile solution containing carprofen, a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class that includes ibuprofen, naproxen, and ketoprofen. Carprofen is the non-proprietary designation for a substituted carbazole, 6-chloro-α-methyl-9H-carbazole-2- acetic acid. The empirical formula is C15H12CINO2 and molecular weight 273.72.
The chemical structure of carprofen is:
Each mL of Carprodyl® Injection contains 50.0 mg carprofen, 30.0 mg L-arginine, 88.5 mg glycocholic acid, 169.0 mg soyphosphatidyl choline, 10.0 mg benzyl alcohol, 7.6 mg sodium hydroxide, with additional sodium hydroxide and hydrochloric acid as needed to adjust pH, and water for injection.
CLINICAL PHARMACOLOGY:
Carprofen is a non-narcotic, non-steroidal anti-inflammatory agent with characteristic analgesic and antipyretic activity approximately equipotent to indomethacin in animal models.1
The mechanism of action of carprofen, like that of other NSAIDs, is believed to be associated with the inhibition of cyclooxygenase activity. Two unique cyclooxygenases have been described in mammals.2 The constitutive cyclooxygenase, COX-1, synthesizes prostaglandins necessary for normal gastrointestinal and renal function. The inducible cyclooxygenase, COX-2, generates prostaglandins involved in inflammation. Inhibition of COX-1 is thought to be associated with gastrointestinal and renal toxicity while inhibition of COX-2 provides anti-inflammatory activity. The specificity of a particular NSAID for COX-2 versus COX-1 may vary from species to species.3 In an in vitro study using canine cell cultures, carprofen demonstrated selective inhibition of COX-2 versus COX-1.4 Clinical relevance of these data has not been shown. Carprofen has also been shown to inhibit the release of several prostaglandins in two inflammatory cell systems: rat polymorphonuclear leukocytes (PMN) and human rheumatoid synovial cells, indicating inhibition of acute (PMN system) and chronic (synovial cell system) inflammatory reactions.1
Several studies have demonstrated that carprofen has modulatory effects on both humoral and cellular immune responses.5-9 Data also indicate that carprofen inhibits the production of osteoclast-activating factor (OAF), PGE1, and PGE2 by its inhibitory effects on prostaglandin biosynthesis.1
Based upon comparison with data obtained from intravenous administration, carprofen is rapidly and nearly completely absorbed (more than 90% bioavailable) when administered orally.10 Peak blood plasma concentrations are achieved in 1 to 3 hours after oral administration of 1, 5, and 25 mg/kg to dogs. The mean terminal half-life of carprofen is approximately 8 hours (range 4.5 to 9.8 hours) after single oral doses varying from 1 to 35 mg/kg of body weight. After a 100 mg single intravenous bolus dose, the mean elimination half-life was approximately 11.7 hours in the dog. Carprofen injection is more than 99% bound to plasma protein and exhibits a very small volume of distribution.
Comparison of a single 25 mg dose in Beagle dogs after subcutaneous and oral administration demonstrated that the dorsoscapular subcutaneous administration results in a slower rate of drug input (as reflected by mean peak observed concentrations) but comparable total drug absorption within a 12 hour dosing interval (as reflected by area under the curve from hours zero to 12 postdose).
Carprofen is eliminated in the dog primarily by biotransformation in the liver followed by rapid excretion of the resulting metabolites (the ester glucuronide of carprofen and the ether glucuronides of 2 phenolic metabolites, 7-hydroxy carprofen and 8-hydroxy carprofen) in the feces (70 to 80%) and urine (10 to 20%). Some enterohepatic circulation of the drug is observed.
INDICATIONS: Carprodyl® Injection is indicated for the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries in dogs.
CONTRAINDICATIONS: Carprodyl® Injection should not be used in dogs exhibiting previous hypersensitivity to carprofen.
WARNINGS: Keep out of reach of children. Not for human use. Consult a physician in cases of accidental human exposure.For use in dogs only. Do not use in cats.
All dogs should undergo a thorough history and physical examination before initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to, and periodically during, administration of any NSAID should be considered. Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions, Animal Safety and Post-Approval Experience).
PRECAUTIONS: As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Effects may result from decreased prostaglandin production and inhibition of the enzyme cyclooxygenase which is responsible for the formation of prostaglandins from arachidonic acid.11-14 When NSAIDs inhibit prostaglandins that cause inflammation they may also inhibit those prostaglandins which maintain normal homeostatic function. These anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease more often than in healthy patients.12,14 NSAID therapy could unmask occult disease which has previously been undiagnosed due to the absence of apparent clinical signs. Patients with underlying renal disease for example, may experience exacerbation or decompensation of their renal disease while on NSAID therapy.11-14 The use of parenteral fluids during surgery should be considered to reduce the potential risk of renal complications when using NSAIDs perioperatively.
Carprofen is an NSAID, and as with others in that class, adverse reactions may occur with its use. The most frequently reported effects have been gastrointestinal signs. Events involving suspected renal, hematologic, neurologic, dermatologic, and hepatic effects have also been reported. Patients at greatest risk for renal toxicity are those that are dehydrated, on concomitant diuretic therapy, or those with renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be approached cautiously, with appropriate monitoring. Concomitant use of Carprodyl® Injection with other anti-inflammatory drugs, such as other NSAlDs or corticosteroids, should be avoided because of the potential increase of adverse reactions, including gastrointestinal ulcerations and/or perforations. Sensitivity to drug-associated adverse reactions varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Carprodyl® Injection treatment was not associated with renal toxicity or gastrointestinal ulceration in well-controlled safety studies of up to ten times the dose in healthy dogs. As with any parenterally injected product, good hygienic procedures should be used when administering carprofen injection. It is suggested to use different sites for additional injections.
Carprodyl® Injection is not recommended for use in dogs with bleeding disorders (e.g., Von Willebrand’s disease), as safety has not been established in dogs with these disorders. The safe use of carprofen injection in animals less than 6 weeks of age, pregnant dogs, dogs used for breeding purposes, or in lactating bitches has not been established. Safety has not been established for IV or IM administration. Studies to determine the activity of carprofen injection when administered concomitantly with other protein-bound or similarly metabolized drugs have not been conducted. Drug compatibility should be monitored closely in patients requiring additional therapy. Such drugs commonly used include cardiac, anticonvulsant and behavioral medications. It has been suggested that treatment with carprofen may reduce the level of inhalant anesthetics needed.15 If additional pain medication is warranted after administration of the total daily dose of carprofen injection, alternative analgesia should be considered. The use of another NSAID is not recommended. Consider appropriate washout times when switching from one NSAID to another or when switching from corticosteroid use to NSAID use.
INFORMATION FOR DOG OWNERS:
Carprodyl® Injection, like other drugs of its class, is not free from adverse reactions.
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include decreased appetite, vomiting, diarrhea, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, yellowing of gums, skin or white of the eye due to jaundice, lethargy, incoordination, seizure, or behavioral changes. Serious adverse reactions associated with this drug class can occur without warning and in rare situations result in death (see Adverse Reactions). Owners should be advised to discontinue Carprodyl® Injection therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is with-drawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID.
ADVERSE REACTIONS: During investigational studies for the caplet formulation, no clinically significant adverse reactions were reported. Some clinical signs were observed during field studies (n=297) which were similar for carprofen and placebo-treated dogs. Incidences of the following were observed in both groups: vomiting (4%), diarrhea (4%), changes in appetite (3%), lethargy (1.4%), behavioral changes (1%), and constipation (0.3%). The product vehicle served as control.
There were no serious adverse events reported during clinical field studies with once daily oral administration of 2 mg/lb. The following categories of abnormal health observations were reported. The product vehicle served as control.
| Percentage of Dogs with Abnormal Health Observations Reported in Clinical Field
Study(2 mg/lb once daily) |
||
| Observation | Carprofen Injection | Placebo |
| (n=129) | (n=132) | |
| Inappetence | 1.6 | 1.5 |
| Vomiting | 3.1 | 3.8 |
| Diarrhea/Soft stool | 3.1 | 4.5 |
| Behavior change | 0.8 | 0.8 |
| Dermatitis | 0.8 | 0.8 |
| PU/PD | 0.8 | -- |
| SAP increase | 7.8 | 8.3 |
| ALT increase | 5.4 | 4.5 |
| AST increase | 2.3 | 0.8 |
| BUN increase | 3.1 | 1.5 |
| Bilirubinuria | 16.3 | 12.1 |
| Ketonuria | 14.7 | 9.1 |
Clinical pathology parameters listed represent reports of increases from pre-treatment values; the use of clinical judgment is necessary to determine clinical relevance (refers also to table below).
There were no serious adverse events reported during clinical field studies for the injectable formulation. The following categories of abnormal health observations were reported. Saline served as placebo control.
|
*A single dog may have experienced more than one occurrence of an event. |
||
| Percentage of Dogs with Abnormal Health Observations Reported in Clinical Field Studies with the Injectable | ||
| Observation* | Carprofen Injection | Placebo |
| (n=168) | (n=163) | |
| Vomiting | 10.1 | 9.2 |
| Diarrhea/Soft stool | 2.4 | 3.7 |
| Dermatitis | 0.6 | 1.2 |
| Dysrhythmia | 0.6 | 0.6 |
| Swelling | 0 | 1.2 |
| Dehiscence | 1.2 | 0 |
| WBC increase | 13.7 | 6.7 |
Post-Approval Experience:
Although not all adverse reactions are reported, the following adverse reactions are based on voluntary post-approval adverse drug experience reporting. The categories of adverse reactions are listed by body system.
Gastrointestinal: Vomiting, diarrhea, constipation, inappetence, melena, hematemesis, gastrointestinal ulceration, gastrointestinal bleeding, pancreatitis.
Hepatic:Inappetence, vomiting, jaundice, acute hepatic toxicity, hepatic enzyme elevation, abnormal liver function test(s), hyperbilirubinemia, bilirubinuria, hypoalbuminemia. Approximately one-fourth of hepatic reports were in Labrador Retrievers.
Neurologic: Ataxia, paresis, paralysis, seizures, vestibular signs, disorientation.
Urinary: Hematuria, polyuria, polydipsia, urinary incontinence, urinary tract infection, azotemia, acute renal failure, tubular abnormalities including acute tubular necrosis, renal tubular acidosis, glucosuria.
Behavioral: Sedation, lethargy, hyperactivity, restlessness, aggressiveness.
Hematologic: Immune-mediated hemolytic anemia, immunemediated thrombocytopenia, blood loss anemia, epistaxis.
Dermatologic: Pruritus, increased shedding, alopecia, pyotraumatic moist dermatitis (hot spots), necrotizing panniculitis/vasculitis, ventral ecchymosis. In rare situations, injection site reactions including necrosis, abscess and seroma formation, and granulomas have been reported with the injectable formulation.
Immunologic or hypersensitivity: Facial swelling, hives, erythema.
In rare situations, death has been associated with some of the adverse reactions listed above.
To report a suspected adverse reaction call 1-800-999-0297.
DOSAGE AND ADMINISTRATION: Carefully consider the potential benefits and risks of Carprodyl® Injection and other treatment options before deciding to use Carprodyl® Injection. Use the lowest effective dose for the shortest duration consistent with individual response. The recommended dosage for subcutaneous administration to dogs is 2 mg/lb (4.4 mg/kg) of body weight daily. The total daily dose may be administered as either 2 mg/lb of body weight once daily or divided and administered as 1 mg/lb (2.2 mg/kg) twice daily. For control of post-operative pain, administer approximately 2 hours before the procedure.
EFFECTIVENESS: Confirmation of the effectiveness of carprofen injection for the relief of pain and inflammation associated with osteoarthritis, and for the control of postoperative pain associated with soft tissue and orthopedic surgeries was demonstrated in 7 placebo-controlled, masked studies examining the anti-inflammatory and analgesic effectiveness of carprofen caplets and injectable in various breeds of dogs.
Separate placebo-controlled, masked, multicenter field studies confirmed the anti-inflammatory and analgesic effectiveness of carprofen caplets when dosed at 2 mg/lb once daily or when divided and administered at 1 mg/lb twice daily. In these two field studies, dogs diagnosed with osteoarthritis showed statistically significant overall improvement based on lameness evaluations by the veterinarian and owner observations when administered carprofen injection at labeled doses.
Based upon the blood level comparison between subcutaneous and oral administration, carprofen injection effectiveness for osteoarthritis after dorsoscapular subcutaneous and oral administration should be similar, although there may be a slight delay in the onset of relief after subcutaneous injection.
Separate placebo-controlled, masked, multicenter field studies confirmed the effectiveness of carprofen injection for the control of postoperative pain when dosed at 2 mg/lb once daily in various breeds of dogs. In these studies, dogs presented for ovariohysterectomy, cruciate repair and aural surgeries were administered carprofen injection preoperatively and for a maximum of 3 days (soft tissue) or 4 days (orthopedic) postoperatively. In general, dogs administered carprofen injection showed statistically significant improvement in pain scores compared to controls.
ANIMAL SAFETY: Laboratory studies in unanesthetized dogs and clinical field studies have demonstrated that carprofen injection is well tolerated in dogs after oral and subcutaneous administration.
In target animal safety studies, carprofen injection was administered orally to healthy Beagle dogs at 1, 3, and 5 mg/lb twice daily (1, 3 and 5 times the recommended total daily dose) for 42 consecutive days with no significant adverse reactions. Serum albumin for a single female dog receiving 5 mg/lb twice daily decreased to 2.1 g/dL after 2 weeks of treatment, returned to the pre-treatment value (2.6 g/dL) after 4 weeks of treatment, and was 2.3 g/dL at the final 6-week evaluation. Over the 6-week treatment period, black or bloody stools were observed in 1 dog (1 incident) treated with 1 mg/lb twice daily and in 1 dog (2 incidents) treated with 3 mg/lb twice daily. Redness of the colonic mucosa was observed in 1 male that received 3 mg/lb twice daily.
Two of 8 dogs receiving 10 mg/lb orally twice daily (10 times the recommended total daily dose) for 14 days exhibited hypoalbuminemia. The mean albumin level in the dogs receiving this dose was lower (2.38 g/dL) than each of 2 placebo control groups (2.88 and 2.93 g/dL, respectively). Three incidents of black or bloody stool were observed in 1 dog. Five of 8 dogs exhibited reddened areas of duodenal mucosa on gross pathologic examination. Histologic examination of these areas revealed no evidence of ulceration, but did show minimal congestion of the lamina propria in 2 of the 5 dogs.
In separate safety studies lasting 13 and 52 weeks, respectively, dogs were administered orally up to 11.4 mg/lb/day (5.7 times the recommended total daily dose of 2 mg/lb) of carprofen. In both studies, the drug was well tolerated clinically by all of the animals. No gross or histologic changes were seen in any of the treated animals. In both studies, dogs receiving the highest doses had average increases in serum L-alanine aminotransferase (ALT) of approximately 20 IU.
In the 52 week study, minor dermatologic changes occurred in dogs in each of the treatment groups but not in the control dogs. The changes were described as slight redness or rash and were diagnosed as non-specific dermatitis. The possibility exists that these mild lesions were treatment related, but no dose relationship was observed.
Clinical field studies were conducted with 549 dogs of different breeds at the recommended oral doses for 14 days (297 dogs were included in a study evaluating 1 mg/lb twice daily and 252 dogs were included in a separate study evaluating 2 mg/lb once daily). In both studies the drug was clinically well tolerated and the incidence of clinical adverse reactions for carprofen injection-treated animals was no higher than placebo-treated animals (placebo contained inactive ingredients found in carprofen injection). For animals receiving 1 mg/lb twice daily, the mean post-treatment serum ALT values were 11 IU greater and 9 IU less than pre-treatment values for dogs receiving carprofen injection and placebo, respectively. Differences were not statistically significant. For animals receiving 2 mg/lb once daily, the mean post-treatment serum ALT values were 4.5 IU greater and 0.9 IU less than pre-treatment values for dogs receiving carprofen injection and placebo, respectively. In the latter study, 3 carprofen injection -treated dogs developed a 3-fold or greater increase in (ALT) and/or (AST) during the course of therapy. One placebo-treated dog had a greater than 2-fold increase in ALT. None of these animals showed clinical signs associated with the laboratory value changes. Changes in clinical laboratory values (hematology and clinical chemistry) were not considered clinically significant. The 1 mg/lb twice daily course of therapy was repeated as needed at 2-week intervals in 244 dogs, some for as long as 5 years.
Clinical field studies were conducted on 331 dogs undergoing orthopedic or soft tissue surgery. Dogs were administered 2 mg/lb of carprofen injection subcutaneously two hours prior to surgery and once daily thereafter, as needed, for 2 days (soft tissue surgery) or 3 days (orthopedic surgery). Carprofen Injection was well tolerated when used in conjunction with a variety of anesthetic-related drugs. The type and severity of abnormal health observations in carprofen injection- and placebo-treated animals were approximately equal and few in number (see Adverse Reactions). The most frequent abnormal health observation was vomiting and was observed at approximately the same frequency in carprofen injection - and placebo-treated animals. Changes in clinicopathologic indices of hematopoetic, renal, hepatic, and clotting function were not clinically significant. The mean post-treatment serum ALT values were 8.4 IU and 7.0 IU less than pre-treatment values for dogs receiving carprofen injection and placebo, respectively. The mean post-treatment AST values were 1.5 IU and 0.7 IU greater for dogs receiving carprofen injection and placebo, respectively.
Swelling and warmth were associated with the injection site after subcutaneous administration of carprofen injection. These findings were not clinically significant. Long term use of the injectable has not been studied.
STORAGE: Store under refrigeration 2°–8°C (36°–46°F). Once broached, product may be stored at temperatures up to 25°C (77°F) for 28 days.
REFERENCES:
- Baruth H, et al: In Anti-Inflammatory and Anti-Rheumatic Drugs, Vol. II, Newer Anti-Inflammatory Drugs, Rainsford KD, ed. CRC Press, Boca Raton, pp. 33–47, 1986.
- Vane JR, Botting RM: Mechanism of action of anti inflammatory drugs. Scand J Rheumatol 25:102, pp. 9-21.
- Grossman CJ, Wiseman J, Lucas FS, et al: Inhibition of constitutive and inducible cyclooxygenase activity in human platelets and mononuclear cells by NSAIDs and COX-2 inhibitors. Inflammation Research 44:253–257, 1995.
- Ricketts AP, Lundy KM, Seibel SB: Evaluation of selective inhibition of canine cyclooxgenase 1 and 2 by carprofen and other nonsteroidal anti-inflammatory drugs. Am J Vet Res 59:11, pp. 1441–1446, November 1998.
- Ceuppens JL, et al: Non-steroidal anti-inflammatory agents inhibit the synthesis of IgM rheumatoid factor in vitro. Lancet 1:528, 1982.
- Ceuppens JL, et al: Endogenous prostaglandin E2 enhances polyclonal immunoglobulin production by ionically inhibiting T suppressor cell activity. Cell Immunol 70:41, 1982.
- Schleimer RP, et al: The effects of prostaglandin synthesis inhibition on the immune response. Immunopharmacology 3:205, 1981.
- Leung KH, et al: Modulation of the development of cell mediated immunity: possible roles of the products of cyclooxgenase and lipoxygenase pathways of arachidonic acid metabolism. Int J Immunopharmacology 4:195, 1982.
- Veit BC: Immunoregulatory activity of cultured-induced suppressor macrophages. Cell Immunol 72:14, 1982.
- Schmitt M, et al: Biopharmaceutical evaluation of carprofen following single intravenous, oral, and rectal doses in dogs. Biopharm Drug Dispos 11(7):585–94, 1990.
- Kore AM: Toxicology of nonsteriodal anti-inflammatory drugs. Veterinary Clinics of North America, Small Animal Practice 20, March 1990.
- Binns SH: Pathogenesis and pathophysiology of ischemic injury in cases of acute renal failure. Compend for Cont Ed 16:1, January 1994.
- Boothe DM: Prostaglandins: Physiology and clinical implications. Compend for Cont Ed 6:11, November 1984.
- Rubin SI: Nonsteroidal anti-inflammatory drugs, prostaglandins, and the kidney. JAVMA 188:9, May 1986. 15.
- Ko CH, Lange DN, Mandsager RE, et al: Effects of butorphanol and carprofen on the minimal alveolar concentration of isoflurane in dogs. JAVMA 217:1025–1028, 2000.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the MSDS/SDS, contact Ceva Animal Health, LLC, at 1-800-999-0297.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth/default.htm
Manufactured For:
Ceva Animal Health, LLC
Lenexa, KS 66215
Made in India
Rev 11/2021
Principal Display Panel – Vial Label
NDC: 13744-507-20
Carprodyl®
(Carprofen)
50 mg/mL Injection
Net contents: 20 mL
Non-steroidal anti-inflammatory drug
For subcutaneous use in dogs only
Caution: Federal law restricts this drug to use by or
on the order of a licensed veterinarian.
Approved by FDA, ANADA 200-593
Principal Display Panel – Carton Label
NDC: 13744-507-20
Carprodyl®
(Carprofen)
50 mg/mL Injection
Net contents: 20 mL
Non-steroidal anti-inflammatory drug
For subcutaneous use in dogs only
Caution: Federal law restricts this drug to use by or
on the order of a licensed veterinarian.
Approved by FDA, ANADA 200-593
| CARPRODYL
carprofen injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ceva Sante Animale (261126049) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Intas Pharmaceuticals Limited | 725927649 | MANUFACTURE, ANALYSIS | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PROCOS SpA | 439262429 | API MANUFACTURE | |
Trademark Results [Carprodyl]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CARPRODYL 87038428 5105037 Live/Registered |
Ceva Santé Animale 2016-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.