SULAR- nisoldipine tablet, film coated, extended release
Sular by
Drug Labeling and Warnings
Sular by is a Prescription medication manufactured, distributed, or labeled by Covis Pharma. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
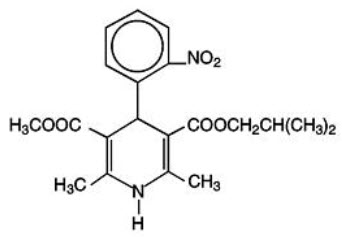
SULAR® (nisoldipine) is an extended release tablet dosage form of the dihydropyridine calcium channel blocker nisoldipine. Nisoldipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, methyl 2-methylpropyl ester, C20H24N2O6, and has the structural formula:
Nisoldipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 388.4. SULAR tablets comprise three layers: a top barrier layer, a middle layer containing nisoldipine, and a bottom barrier layer. The erodible barrier layers and the hydrogel middle layer provide for the controlled release of the drug. SULAR tablets contain either 8.5, 17, or 34 mg of nisoldipine for once-a-day oral administration.
Inactive ingredients in the formulation include: Hypromellose, hypromellose phthalate, lactose, glyceryl behenate, povidone, magnesium stearate, silicon dioxide, methacrylic acid copolymer, and sodium lauryl sulfate. Inactive ingredients in the film coating include: polydextrose, titanium dioxide, hypromellose, polyethylene glycol, iron oxide, and carnauba wax. Additionally, the 17 mg formulation contains FD&C Yellow #5.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Nisoldipine is a member of the dihydropyridine class of calcium channel antagonists (calcium ion antagonists or slow channel blockers) that inhibit the transmembrane influx of calcium into vascular smooth muscle and cardiac muscle. It reversibly competes with other dihydropyridines for binding to the calcium channel. Because the contractile process of vascular smooth muscle is dependent upon the movement of extracellular calcium into the muscle through specific ion channels, inhibition of the calcium channel results in dilation of the arterioles. In vitro studies show that the effects of nisoldipine on contractile processes are selective, with greater potency on vascular smooth muscle than on cardiac muscle. Although, like other dihydropyridine calcium channel blockers, nisoldipine has negative inotropic effects in vitro, studies conducted in intact anesthetized animals have shown that the vasodilating effect occurs at doses lower than those that affect cardiac contractility.
The effect of nisoldipine on blood pressure is principally a consequence of a dose-related decrease of peripheral vascular resistance. While nisoldipine, like other dihydropyridines, exhibits a mild diuretic effect, most of the antihypertensive activity is attributed to its effect on peripheral vascular resistance.
Pharmacokinetics and Metabolism
Nisoldipine pharmacokinetics are independent of the dose across the clinical dosage range of 17 to 51 mg, with plasma concentrations proportional to dose. Nisoldipine accumulation, during multiple dosing, is predictable from a single dose. Nisoldipine is relatively well absorbed into the systemic circulation with 87% of the radiolabeled drug recovered in urine and feces. The absolute bioavailability of nisoldipine is about 5%. Nisoldipine's low bioavailability is due, in part, to pre-systemic metabolism in the gut wall, and this metabolism decreases from the proximal to the distal parts of the intestine. A pronounced food-effect is observed when SULAR is administered with a high-fat meal resulting in an increased peak concentration (Cmax) of up to 245%. Total exposure (AUC) is decreased by 25%. As a result, SULAR should be taken on an empty stomach (1 hour before or 2 hours after a meal).
Maximal plasma concentrations of nisoldipine are reached at 9.2 ± 5.1 hours. The terminal elimination half-life (reflecting post absorption clearance of nisoldipine) ranges from 13.7 ± 4.3 hours. After oral administration, the concentration of (+)-nisoldipine, the active enantiomer, is about 6 times higher than the inactive (-) -nisoldipine enantiomer. The plasma protein binding of nisoldipine is very high, with less than 1% unbound over the plasma concentration range of 100 ng/mL to 10 mcg/mL.
Nisoldipine is highly metabolized; 5 major urinary metabolites have been identified. Although 60 - 80% of an oral dose undergoes urinary excretion, only traces of unchanged nisoldipine are found in urine. The major biotransformation pathway appears to be the hydroxylation of the isobutyl ester. A hydroxylated derivative of the side chain, present in plasma at concentrations approximately equal to the parent compound, appears to be the only active metabolite, and has about 10% of the activity of the parent compound. Cytochrome P450 enzymes are believed to play a major role in the metabolism of nisoldipine. The particular isoenzyme system responsible for its metabolism has not been identified, but other dihydropyridines are metabolized by cytochrome P450 IIIA4. Nisoldipine should not be administered with grapefruit juice, as this has been shown, in a study of 12 subjects, to interfere with nisoldipine metabolism, resulting in a mean increase in Cmax of about 3-fold (ranging up to about 7-fold) and AUC of almost 2-fold (ranging up to about 5-fold). A similar phenomenon has been seen with several other dihydropyridine calcium channel blockers.
Special Populations
Renal Dysfunction
Because renal elimination is not an important pathway, bioavailability and pharmacokinetics of SULAR were not significantly different in patients with various degrees of renal impairment. Dosing adjustments in patients with mild to moderate renal impairment are not necessary.
Geriatric
Elderly patients have been found to have 2 to 3 fold higher plasma concentrations (Cmax and AUC) than young subjects. This should be reflected in more cautious dosing (see DOSAGE AND ADMINISTRATION).
Hepatic Insufficiency
In patients with liver cirrhosis given a dose bioequivalent to 8.5 mg SULAR, plasma concentrations of the parent compound were 4 to 5 times higher than those in healthy young subjects. Lower starting and maintenance doses should be used in cirrhotic patients (see DOSAGE AND ADMINISTRATION).
Gender and Race
The effect of gender or race on the pharmacokinetics of nisoldipine has not been investigated.
Disease States
Hypertension does not significantly alter the pharmacokinetics of nisoldipine.
Pharmacodynamics
Hemodynamic Effects
Administration of a single dose of nisoldipine leads to decreased systemic vascular resistance and blood pressure with a transient increase in heart rate. The change in heart rate is greater with immediate release nisoldipine preparations. The effect on blood pressure is directly related to the initial degree of elevation above normal. Chronic administration of nisoldipine results in a sustained decrease in vascular resistance and small increases in stroke index and left ventricular ejection fraction. A study of the immediate release formulation showed no effect of nisoldipine on the renin-angiotensin-aldosterone system or on plasma norepinephrine concentration in normals. Changes in blood pressure in hypertensive patients given SULAR were dose related over the clinical dosage range.
Nisoldipine does not appear to have significant negative inotropic activity in intact animals or humans, and did not lead to worsening of clinical heart failure in three small studies of patients with asymptomatic and symptomatic left ventricular dysfunction. There is little information, however, in patients with severe congestive heart failure, and all calcium channel blockers should be used with caution in any patient with heart failure.
Electrophysiologic Effects
Nisoldipine has no clinically important chronotropic effects. Except for mild shortening of sinus cycle, SA conduction time and AH intervals, single oral doses up to 20 mg of immediate release nisoldipine did not significantly change other conduction parameters. Similar electrophysiologic effects were seen with single IV doses, which could be blunted in patients pre-treated with beta-blockers. Dose and plasma level related flattening or inversion of T-waves have been observed in a few small studies. Such reports were concentrated in patients receiving rapidly increased high doses in one study; the phenomenon has not been a cause of safety concern in large clinical trials.
Clinical Studies in Hypertension
The antihypertensive efficacy of SULAR was studied in 5 double-blind, placebo-controlled, randomized studies, in which over 600 patients were treated with SULAR as monotherapy and about 300 with placebo; 4 of the five studies compared 2 or 3 fixed doses while the fifth allowed titration from doses bioequivalent to 8.5 - 34 mg. Once daily administration of SULAR produced sustained reductions in systolic and diastolic blood pressures over the 24 hour dosing interval in both supine and standing positions. The mean placebo-subtracted reductions in supine systolic and diastolic blood pressure at trough, 24 hours post-dose, in these studies, are shown below. Changes in standing blood pressure were similar:
MEAN SUPINE TROUGH SYSTOLIC AND DIASTOLIC BLOOD PRESSURE CHANGES (mm Hg) SULAR
Doses bioequivalent to:
(mg/day)8.5 mg
17 mg
25.5 mg
34 mg
8.5-34 mg
titratedSystolic
8
11
11
14
15
Diastolic
3
5
7
7
8
In patients receiving atenolol, supine blood pressure reductions with SULAR at doses bioequivalent to 17 and 34 mg once daily were 12/6 and 19/8 mm Hg, respectively. The sustained antihypertensive effect of SULAR was demonstrated by 24 hour blood pressure monitoring and examination of peak and trough effects. The trough/peak ratios ranged from 70 to 100% for diastolic and systolic blood pressure. The mean change in heart rate in these studies was less than one beat per minute. In 4 of the 5 studies, patients received initial doses bioequivalent to 17-25.5 mg SULAR without incident (excessive effects on blood pressure or heart rate). The fifth study started patients on lower doses of SULAR.
Patient race and gender did not influence the blood pressure lowering effect of SULAR. Despite the higher plasma concentration of nisoldipine in the elderly, there was no consistent difference in their blood pressure response except that the lowest clinical dose was somewhat more effective than in non-elderly patients. No postural effect on blood pressure was apparent and there was no evidence of tolerance to the antihypertensive effect of SULAR in patients treated for up to one year.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Increased angina and/or myocardial infarction in patients with coronary artery disease:
Rarely, patients, particularly those with severe obstructive coronary artery disease, have developed increased frequency, duration and/or severity of angina, or acute myocardial infarction on starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been established. In controlled studies of SULAR in patients with angina this was seen about 1.5% of the time in patients given nisoldipine, compared with 0.9% in patients given placebo.
-
PRECAUTIONS
General
Hypotension
Because nisoldipine, like other vasodilators, decreases peripheral vascular resistance, careful monitoring of blood pressure during the initial administration and titration of SULAR is recommended. Close observation is especially important for patients already taking medications that are known to lower blood pressure. Although in most patients the hypotensive effect of SULAR is modest and well tolerated, occasional patients have had excessive and poorly tolerated hypotension. These responses have usually occurred during initial titration or at the time of subsequent upward dosage adjustment.
Congestive Heart Failure
Although acute hemodynamic studies of nisoldipine in patients with NYHA Class II-IV heart failure have not demonstrated negative inotropic effects, safety of SULAR in patients with heart failure has not been established. Caution therefore should be exercised when using SULAR in patients with heart failure or compromised ventricular function, particularly in combination with a beta-blocker.
Patients with Hepatic Impairment
Because nisoldipine is extensively metabolized by the liver and, in patients with cirrhosis, it reaches blood concentrations about 5 times those in normals, SULAR should be administered cautiously in patients with severe hepatic dysfunction (see DOSAGE AND ADMINISTRATION).
Information for Patients
SULAR is an extended release tablet and should be swallowed whole. Tablets should not be chewed, divided or crushed. SULAR should be taken on an empty stomach (1 hour before or 2 hours after a meal). Grapefruit juice, which has been shown to increase significantly the bioavailability of nisoldipine and other dihydropyridine type calcium channel blockers, should not be taken with SULAR.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Drug Interactions
A 30 to 45% increase in AUC and Cmax of nisoldipine was observed with concomitant administration of cimetidine 400 mg twice daily. Ranitidine 150 mg twice daily did not interact significantly with nisoldipine (AUC was decreased by 15 - 20%). No pharmacodynamic effects of either histamine H2 receptor antagonist were observed.
CYP3A4 inhibitors and inducers
SULAR is substrate of CYP3A4 and coadministration of SULAR with any known inducer or inhibitor of CYP3A4 should be avoided in general.
Coadministration of phenytoin with a dose bioequivalent to 34 mg SULAR tablets in epileptic patients lowered the nisoldipine plasma concentrations to undetectable levels. Coadministration of SULAR with phenytoin should be avoided and alternative antihypertensive therapy should be considered. Pharmacokinetic interactions between nisoldipine and beta-blockers (atenolol, propranolol) were variable and not significant. Propranolol attenuated the heart rate increase following administration of immediate release nisoldipine. The blood pressure effect of SULAR tended to be greater in patients on atenolol than in patients on no other antihypertensive therapy. Quinidine at 648 mg bid decreased the bioavailability (AUC) of nisoldipine by 26%, but not the peak concentration. Immediate release nisoldipine increased plasma quinidine concentrations by about 20%. This interaction was not accompanied by ECG changes and its clinical significance is not known. No significant interactions were found between nisoldipine and warfarin or digoxin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Dietary administration of nisoldipine to male and female rats for up to 24 months (mean doses up to 82 and 111 mg/kg/day, 16 and 19 times the maximum recommended human dose [MRHD] on a mg/m2 basis, respectively) and female mice for up to 21 months (mean doses of up to 217 mg/kg/day, 20 times the MRHD on a mg/m2 basis) revealed no evidence of tumorigenic effect of nisoldipine. In male mice receiving a mean dose of 163 mg nisoldipine/kg/day (16 times the MRHD of 60 mg/day on a mg/m2 basis), an increased frequency of stomach papilloma, but still within the historical range, was observed. No evidence of stomach neoplasia was observed at lower doses (up to 58 mg/kg/day). Nisoldipine was negative when tested in a battery of genotoxicity assays including the Ames test and the CHO/HGRPT assay for mutagenicity and the in vivo mouse micronucleus test and in vitro CHO cell test for clastogenicity.
When administered to male and female rats at doses of up to 30 mg/kg/day (about 5 times the MRHD on a mg/m2 basis) nisoldipine had no effect on fertility.
Pregnancy
Nisoldipine was neither teratogenic nor fetotoxic at doses that were not maternally toxic. Nisoldipine was fetotoxic but not teratogenic in rats and rabbits at doses resulting in maternal toxicity (reduced maternal body weight gain). In pregnant rats, increased fetal resorption (postimplantation loss) was observed at 100 mg/kg/day and decreased fetal weight was observed at both 30 and 100 mg/kg/day. These doses are, respectively, about 5 and 16 times the MRHD when compared on a mg/m2 basis. In pregnant rabbits, decreased fetal and placental weights were observed at a dose of 30 mg/kg/day, about 10 times the MRHD when compared on a mg/m2 basis. In a study in which pregnant monkeys (both treated and control) had high rates of abortion and mortality, the only surviving fetus from a group exposed to a maternal dose of 100 mg nisoldipine/kg/day (about 30 times the MRHD when compared on a mg/m2 basis) presented with forelimb and vertebral abnormalities not previously seen in control monkeys of the same strain. There are no adequate and well controlled studies in pregnant women. SULAR should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether nisoldipine is excreted in human milk. Because many drugs are excreted in human milk, a decision should be made to discontinue nursing, or to discontinue SULAR, taking into account the importance of the drug to the mother.
Geriatric Use
Clinical studies of nisoldipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Patients over 65 are expected to develop higher plasma concentrations of nisoldipine. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE EXPERIENCES
More than 6000 patients world-wide have received nisoldipine in clinical trials for the treatment of hypertension, either as the immediate release or the SULAR extended release formulation. Of about 1,500 patients who received SULAR in hypertension studies, about 55% were exposed for at least 2 months and about one third were exposed for over 6 months, the great majority at doses equivalent to 17 mg and above.
SULAR is generally well-tolerated. In the U.S. clinical trials of SULAR in hypertension, 10.9% of the 921 SULAR patients discontinued treatment due to adverse events compared with 2.9% of 280 placebo patients. The frequency of discontinuations due to adverse experiences was related to dose, with a 5.4% and 10.9% discontinuation rate at the lowest and highest daily dose, respectively.
The most frequently occurring adverse experiences with SULAR are those related to its vasodilator properties; these are generally mild and only occasionally lead to patient withdrawal from treatment. The table below, from U.S. placebo-controlled parallel dose response trials of SULAR using doses across the clinical dosage range in patients with hypertension, lists all of the adverse events, regardless of the causal relationship to SULAR, for which the overall incidence on SULAR was both >1% and greater with SULAR than with placebo.
Only peripheral edema and possibly dizziness appear to be dose related. Adverse Event
Nisoldipine (%)
(n=663)Placebo (%)
(n=280)Peripheral Edema
22
10
Headache
22
15
Dizziness
5
4
Pharyngitis
5
4
Vasodilation
4
2
Sinusitis
3
2
Palpitation
3
1
Chest Pain
2
1
Nausea
2
1
Rash
2
1
Adverse Event
SULAR, dose bioequivalent to:
Placebo
8.5mg
17mg
25.5mg
34mg
(Rates in %)
N=280
N=30
N=170
N=105
N=139
Peripheral Edema
10
7
15
20
27
Dizziness
4
7
3
3
4
The common adverse events occurred at about the same rate in men as in women, and at a similar rate in patients over age 65 as in those under that age, except that headache was much less common in older patients. Except for peripheral edema and vasodilation, which were more common in whites, adverse event rates were similar in blacks and whites.
The following adverse events occurred in ≤1% of all patients treated for hypertension in U.S. and foreign clinical trials, or with unspecified incidence in other studies. Although a causal relationship of SULAR to these events cannot be established, they are listed to alert the physician to a possible relationship with SULAR treatment.
Body As A Whole: cellulitis, chills, facial edema, fever, flu syndrome, malaise
Cardiovascular: atrial fibrillation, cerebrovascular accident, congestive heart failure, first degree AV block, hypertension, hypotension, jugular venous distension, migraine, myocardial infarction, postural hypotension, ventricular extrasystoles, supraventricular tachycardia, syncope, systolic ejection murmur, T wave abnormalities on ECG (flattening, inversion, nonspecific changes), venous insufficiency
Digestive: abnormal liver function tests, anorexia, colitis, diarrhea, dry mouth, dyspepsia, dysphagia, flatulence, gastritis, gastrointestinal hemorrhage, gingival hyperplasia, glossitis, hepatomegaly, increased appetite, melena, mouth ulceration
Endocrine: diabetes mellitus, thyroiditis
Hemic and Lymphatic: anemia, ecchymoses, leukopenia, petechiae
Metabolic and Nutritional: gout, hypokalemia, increased serum creatine kinase, increased nonprotein nitrogen, weight gain, weight loss
Musculoskeletal: arthralgia, arthritis, leg cramps, myalgia, myasthenia, myositis, tenosynovitis
Nervous: abnormal dreams, abnormal thinking and confusion, amnesia, anxiety, ataxia, cerebral ischemia, decreased libido, depression, hypesthesia, hypertonia, insomnia, nervousness, paresthesia, somnolence, tremor, vertigo
Respiratory: asthma, dyspnea, end inspiratory wheeze and fine rales, epistaxis, increased cough, laryngitis, pharyngitis, pleural effusion, rhinitis, sinusitis
Skin and Appendages: acne, alopecia, dry skin, exfoliative dermatitis, fungal dermatitis, herpes simplex, herpes zoster, maculopapular rash, pruritus, pustular rash, skin discoloration, skin ulcer, sweating, urticaria
Special Senses: abnormal vision, amblyopia, blepharitis, conjunctivitis, ear pain, glaucoma, itchy eyes, keratoconjunctivitis, otitis media, retinal detachment, tinnitus, watery eyes, taste disturbance, temporary unilateral loss of vision, vitreous floater
Urogenital: dysuria, hematuria, impotence, nocturia, urinary frequency, increased BUN and serum creatinine, vaginal hemorrhage, vaginitis
The following postmarketing event has been reported very rarely in patients receiving SULAR: systemic hypersensitivity reaction which may include one or more of the following; angioedema, shortness of breath, tachycardia, chest tightness, hypotension, and rash. A definite causal relationship with SULAR has not been established. An unusual event observed with immediate release nisoldipine but not observed with SULAR was one case of photosensitivity. Gynecomastia has been associated with the use of calcium channel blockers.
-
OVERDOSAGE
There is no experience with nisoldipine overdosage. Generally, overdosage with other dihydropyridines leading to pronounced hypotension calls for active cardiovascular support including monitoring of cardiovascular and respiratory function, elevation of extremities, judicious use of calcium infusion, pressor agents and fluids. Clearance of nisoldipine would be expected to be slowed in patients with impaired liver function. Since nisoldipine is highly protein bound, dialysis is not likely to be of any benefit; however, plasmapheresis may be beneficial.
-
DOSAGE AND ADMINISTRATION
The dosage of SULAR must be adjusted to each patient's needs. Therapy usually should be initiated with 17 mg orally once daily, then increased by 8.5 mg per week or longer intervals, to attain adequate control of blood pressure. Usual maintenance dosage is 17 to 34 mg once daily. Blood pressure response increases over the 8.5 - 34 mg daily dose range but adverse event rates also increase. Doses beyond 34 mg once daily are not recommended. SULAR has been used safely with diuretics, ACE inhibitors, and beta-blocking agents. Patients over age 65, or patients with impaired liver function, are expected to develop higher plasma concentrations of nisoldipine. Their blood pressure should be monitored closely during any dosage adjustment. A starting dose not exceeding 8.5 mg daily is recommended in these patient groups. SULAR tablets should be administered orally once daily. SULAR should be taken on an empty stomach (1 hour before or 2 hours after a meal). Grapefruit products should be avoided before and after dosing. SULAR is an extended release dosage form and tablets should be swallowed whole, not bitten, divided or crushed.
-
HOW SUPPLIED
SULAR extended release tablets are supplied as 8.5 mg and 17 mg round film coated tablets and 34 mg elliptic film coated tablets. The different strengths can be identified as follows:
Strength
Color
Markings
8.5 mg
Oyster
SCI 500
17 mg
Yellow Cream
SCI 501
34 mg
Burnt Orange
SCI 503
SULAR Tablets are supplied in bottles of 100:
NDC Code
Strength
70515-500-10
8.5 mg
70515-501-10
17 mg
70515-503-10
34 mg
Protect from light and moisture. Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Dispense in tight, light-resistant containers.
Rx only
Sular is a trademark of Covis Pharma
©2017 Covis Pharma.Manufactured for:
Covis Pharma
Zug, 6300 SwitzerlandMade in Germany
Rev 07/17
-
PRINCIPAL DISPLAY PANEL - 8.5 mg Carton
NDC: 70515-500-10
100 Tablets
Sular®
(nisoldipine)
Extended Release
Tablets8.5 mg
Rx only
See package insert for full prescribing
information.COVIS
Store at 20°-25°C (68°-77°F); excursions
permitted between 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature].
Protect from light and moisture. Dispense
in USP tight, light-resistant container.See package insert for full prescribing
information.Manufactured for:
Covis Pharma
Zug, 6300 SwitzerlandMade in Germany
GTIN: XXXXXXXXXXXXXX
S/N: XXXXXXXXXXXX
EXP: MMM/YYYY
LOT: XXXXXXX -
PRINCIPAL DISPLAY PANEL - 17 mg Carton
NDC: 70515-501-10
100 Tablets
Sular®
(nisoldipine)
Extended Release
Tablets17 mg
Rx only
See package insert for full prescribing
information.COVIS
Store at 20°-25°C (68°-77°F); excursions
permitted between 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature].
Protect from light and moisture. Dispense
in USP tight, light-resistant container.See package insert for full prescribing
information.Manufactured for:
Covis Pharma
Zug, 6300 SwitzerlandMade in Germany
GTIN: XXXXXXXXXXXXXX
S/N: XXXXXXXXXXXX
EXP: MMM/YYYY
LOT: XXXXXXX -
PRINCIPAL DISPLAY PANEL - 34 mg Carton
NDC: 70515-503-10
100 Tablets
Sular®
(nisoldipine)
Extended Release
Tablets34 mg
Rx only
See package insert for full prescribing
information.COVIS
Store at 20°-25°C (68°-77°F); excursions
permitted between 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature].
Protect from light and moisture. Dispense
in USP tight, light-resistant container.See package insert for full prescribing
information.Manufactured for:
Covis Pharma
Zug, 6300 SwitzerlandMade in Germany
GTIN: XXXXXXXXXXXXXX
S/N: XXXXXXXXXXXX
EXP: MMM/YYYY
LOT: XXXXXXX -
INGREDIENTS AND APPEARANCE
SULAR
nisoldipine tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70515-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NISOLDIPINE (UNII: 4I8HAB65SZ) (NISOLDIPINE - UNII:4I8HAB65SZ) NISOLDIPINE 8.5 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color GRAY (Oyster) Score no score Shape ROUND Size 7mm Flavor Imprint Code SCI;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70515-500-10 1 in 1 CARTON 02/03/2017 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020356 02/03/2017 SULAR
nisoldipine tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70515-501 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NISOLDIPINE (UNII: 4I8HAB65SZ) (NISOLDIPINE - UNII:4I8HAB65SZ) NISOLDIPINE 17 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color YELLOW (Yellow Cream) Score no score Shape ROUND Size 8mm Flavor Imprint Code SCI;501 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70515-501-10 1 in 1 CARTON 02/03/2017 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020356 02/03/2017 SULAR
nisoldipine tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70515-503 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NISOLDIPINE (UNII: 4I8HAB65SZ) (NISOLDIPINE - UNII:4I8HAB65SZ) NISOLDIPINE 34 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:2) (UNII: 5KY68S2577) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color ORANGE (Burnt Orange) Score no score Shape OVAL (Elliptic) Size 15mm Flavor Imprint Code SCI;503 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70515-503-10 1 in 1 CARTON 02/03/2017 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020356 02/03/2017 Labeler - Covis Pharma (486209070)
Trademark Results [Sular]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SULAR 75025753 2175325 Live/Registered |
COVIS PHARMA B.V. 1995-11-30 |
 SULAR 74322049 1939088 Live/Registered |
COVIS PHARMA B.V. 1992-10-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.