KLISYRI- tirbanibulin ointment
Klisyri by
Drug Labeling and Warnings
Klisyri by is a Prescription medication manufactured, distributed, or labeled by Almirall, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use KLISYRI safely and effectively. See full prescribing information for KLISYRI.
KLISYRI (tirbanibulin) ointment, for topical use
Initial U.S. Approval: 2020
INDICATIONS AND USAGE
KLISYRI is a microtubule inhibitor indicated for the topical treatment of actinic keratosis of the face or scalp. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Ointment: 1% tirbanibulin supplied in unit-dose packets. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Ophthalmic Adverse Reactions: May cause eye irritation upon ocular exposure. Avoid transfer of the drug into the eyes and to the periocular area. If accidental exposure occurs, flush eyes with water and seek medical care. (5.1)
- Local Skin Reactions: Local skin reactions can occur including severe reactions (e.g., vesiculation/pustulation, erosion/ulceration) in the treated area. Avoid use until skin is healed from any previous drug or surgical treatment. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2%) are local skin reactions, application site pruritus, and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Almirall, at 1-866-665-2782 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ophthalmic Adverse Reactions
5.2 Local Skin Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For topical use only; not for oral or ophthalmic use.
Apply KLISYRI evenly to cover up to 100 cm2 treatment field on the face or balding scalp once daily for 5 consecutive days using 1 unit-dose packet per application.
Wash hands immediately with soap and water after application.
Avoid washing and touching the treated area for approximately 8 hours after application of KLISYRI. Following this time, the area may be washed with a mild soap.
Avoid transfer of KLISYRI to the periocular area [see Warnings and Precautions (5.1)].
Avoid application near and around the mouth and lips.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Ophthalmic Adverse Reactions
KLISYRI may cause eye irritation.
Avoid transfer of the drug into the eyes and to the periocular area during and after application. Wash hands immediately after application. If accidental exposure occurs, instruct patient to flush eyes with water and seek medical care as soon as possible.
5.2 Local Skin Reactions
Local skin reactions, including severe reactions (erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation and erosion/ulceration) in the treated area can occur after topical application of KLISYRI [see Adverse Reactions (6.1)]. Occlusion after topical application of KLISYRI is more likely to result in irritation. Avoid use until skin is healed from any previous drug, procedure, or surgical treatment.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Two double-blind, vehicle-controlled clinical trials were conducted in 702 adult subjects with actinic keratosis on the face or scalp. Subjects were randomized 1:1 to KLISYRI or vehicle. Subjects enrolled in the trials had 4 to 8 clinically typical, visible, and discrete AK lesions in a contiguous area of 25 cm2 on the face or scalp. Subjects’ average age was 70 years (range 45 to 96 years) and they were predominantly White (99%), male (87%), with Fitzpatrick skin types I or II (72%) and actinic keratosis on the face (68%) or scalp (32%). Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp.
In the controlled trials, local skin reactions (LSRs) were collected independent of adverse events. Local skin reactions including erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, erosions/ulcerations were assessed by the investigators using a grading scale of 0 = absent, 1 = mild (slightly, barely perceptible), 2 = moderate (distinct presence), and 3 = severe (marked, intense).
The percentages of subjects with the maximal post-baseline grades for each local skin reaction (LSR) greater than baseline by treatment group are provided in Table 1. LSRs were mostly mild to moderate in severity (Table 1).
Table 1 Post-Baseline Local Skin Reactions in the Treatment Area (face or scalp) - Pooled Data from 2 Controlled Clinical Phase 3 Trials KLISYRI
N = 353Vehicle
N = 349Local Skin Reactions Mild
n (%)Moderate
n (%)Severe
n (%)Mild
n (%)Moderate
n (%)Severe
n (%)Erythema 76 (22%) 223 (63%) 22 (6%) 98 (28%) 20 (6%) 0 Flaking/ Scaling 92 (26%) 166 (47%) 31 (9%) 86 (25%) 33 (9%) 1 (<1%) Crusting 107 (30%) 50 (14%) 7 (2%) 31 (9%) 8 (2%) 0 Swelling 102 (29%) 32 (9%) 2 (<1%) 15 (4%) 1 (<1%) 0 Vesiculation/ Pustulation 25 (7%) 2 (<1%) 2 (<1%) 3 (<1%) 0 0 Erosion/ Ulceration 32 (9%) 9 (3%) 0 10 (3%) 0 0 Table 2 presents the adverse reactions experienced in ≥2% of subjects participating in the controlled clinical trials with KLISYRI. No subject withdrew from the trials due to adverse reactions.
Table 2 Adverse Reactions Occurring in ≥2% of Subjects in 2 Controlled Clinical Trials– Pooled Safety Population a Application site pain includes pain, tenderness, stinging, and burning sensation at the application site.
Adverse Reaction
System Organ ClassKLISYRI
N = 353Vehicle
N = 349Number of Subjects (%) with any adverse reaction (possibly related to treatment) 56 (16%) 35 (10%) Application site pruritus 32 (9%) 21 (6%) Application site paina 35 (10%) 11 (3%) For the 51 subjects (45 KLISYRI, 6 vehicle) who maintained complete clearance through the 12-month follow-up period, no additional local adverse reactions were reported.
In a multicenter, open-label safety trial of 105 subjects where KLISYRI was applied to a treatment field of 100 cm2 on the face or balding scalp, the results were comparable to the safety profile established by the controlled trials in subjects with a 25 cm2 treatment area.
Dermal Safety Studies
Clinical studies in healthy subjects demonstrated KLISYRI did not cause contact sensitization (261 subjects), phototoxic skin reactions (31 subjects), or photoallergic skin reactions (64 subjects).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with KLISYRI use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
In animal reproduction studies, oral administration of tirbanibulin to pregnant rats during the period of organogenesis resulted in an increased incidence of fetal deaths and malformations at a systemic exposure that was at least 19 times the exposure associated with the maximum recommended human dose (MRHD). Oral administration of tirbanibulin to pregnant rabbits during the period of organogenesis resulted in reduced mean fetal weight and size at a systemic exposure that was 41 times the exposure associated with the MRHD (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
Tirbanibulin induced fetal deaths and external, visceral, and skeletal malformations when administered orally to pregnant rats during the period of organogenesis at doses greater than or equal to 1.25 mg/kg/day, which resulted in systemic exposures at least 19 times the exposure associated with the MRHD on an Area Under the Curve (AUC) comparison basis. Tirbanibulin had no apparent effects on fetal development in rats at a dose of 0.5 mg/kg/day, which resulted in systemic exposures 5 times the exposure associated with the MRHD.
Tirbanibulin reduced mean fetal weight and size (crown-rump length) when administered orally to pregnant rabbits during the period of organogenesis at a dose of 3 mg/kg/day, which resulted in a systemic exposure 41 times the exposure associated with the MRHD on an AUC comparison basis. Tirbanibulin had no apparent effects on fetal development in rabbits at a dose of 1 mg/kg/day, which resulted in systemic exposures 14 times the exposure associated with the MRHD.
Tirbanibulin was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats during the period of organogenesis through lactation at dosages up to 1.25 mg/kg/day. These dosages resulted in systemic exposures up to 19 times the exposure associated with the MRHD on an AUC comparison basis. No adverse effects on maternal function or developmental, neurobehavioral, or reproductive performance of offspring were observed.
8.2 Lactation
Risk Summary
There are no data on lactational transfer of KLISYRI to human or animal milk. The effects of KLISYRI on the breastfed infant, or its effects on milk production, are unknown.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KLISYRI and any potential adverse effects on the breastfed child from tirbanibulin or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of KLISYRI for actinic keratosis in subjects less than 18 years of age have not been established. Actinic keratosis is not a condition generally seen within the pediatric population.
8.5 Geriatric Use
Of the 353 subjects with AK treated with KLISYRI in the 2 controlled Phase 3 trials, 246 (70%) were 65 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
KLISYRI (tirbanibulin) ointment is a microtubule inhibitor for topical use. The chemical name of tirbanibulin is N-benzyl-2-(5-(4-(2-morpholinoethoxy)phenyl)pyridin-2-yl) acetamide. The molecular weight is 431.4 and the molecular formula is C26H29N3O3. Tirbanibulin’s structural formula is:
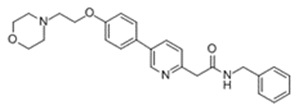
Tirbanibulin ointment 1% contains 10 mg tirbanibulin per gram of white to off-white ointment containing mono- and di-glycerides and propylene glycol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tirbanibulin is a microtubule inhibitor. The mechanism of action of KLISYRI for the topical treatment of actinic keratosis is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of tirbanibulin in the treatment of actinic keratosis is unknown.
12.3 Pharmacokinetics
Absorption
Following topical treatment of a mean daily dose of 348 mg (range: 224 to 435 mg) of KLISYRI to a 100 cm2 contiguous area of the face or balding scalp, once daily for 5 consecutive days. On Day 5, systemic exposure to tirbanibulin had a mean±SD maximum plasma concentration (Cmax) of 1.32±0.74 ng/mL and 0.71±0.31 ng/mL, and a mean±SD area under the plasma concentration from time zero to 24 hours (AUC24) of 19.6±8.1 h∗ng/mL and 11.7±4.4 h∗ng/mL, in subjects who received the face and scalp topical treatment, respectively. The median time to reach Cmax (Tmax) was ~6 hours.
Distribution
Plasma protein binding of tirbanibulin is 88% and is independent of concentrations in the range of 0.01 to 10 µg/mL.
Elimination
Metabolism
Following topical treatment with KLISYRI to adult subjects with actinic keratosis, the plasma concentrations of KX2-5036, KX2-5163, and KX2-5180, three pharmacologically inactive metabolites, were detectable with the highest plasma concentrations of 0.36 ng/mL, 0.42 ng/mL, and 1.70 ng/mL, respectively.
The in vitro study indicated that incubation of 1 or 10 µM tirbanibulin with human hepatocytes generated KX2-5036, KX2-5162 and other unidentified metabolites.
In vitro, tirbanibulin is mainly metabolized by CYP3A4, and to a lesser extent, CYP2C8.
Excretion
Excretion of tirbanibulin has not been fully characterized in humans.
Drug Interactions
Clinical Studies
No clinical studies evaluating the drug interaction potential of KLISYRI have been conducted.
In Vitro Studies
CYP Enzymes: Tirbanibulin and the metabolite KX2-5036 directly or time-dependently inhibited CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4 with an IC50 value of >17 µM. Tirbanibulin up to 1 µM (431.5 ng/mL) and the metabolite KX2-5036 up to 3 µM (1024 ng/mL) did not induce CYP 1A2, 2B6, or 3A4. The metabolite KX2-5180 was neither an inhibitor of CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 or 3A4 with an IC50 value of > 225 nM (85 ng/mL) nor an inducer of CYP1A2, 2B6, and 3A4 at a concentration of 225 nM (85 ng/mL). These findings suggest that KLISYRI has no clinically meaningful effect on the PK of drugs metabolized by CYP 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4.
Drug Transporters: Neither tirbanibulin nor the metabolite KX2-5036 was a substrate of MDR1, BCRP, BSEP, MRP2, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OATP1B3, OCT1 or OCT2. The metabolite KX2-5180 was a substrate of BCRP, but not a substrate for other transporters. Tirbanibulin and the metabolites of KX2-5036 and KX2-5180 inhibited MDR1, BCRP, MRP2, BSEP, MATE1, MATE2-K, OAT1, OTA3, OATP1B1, OATP1B3, OCT1 and/or OCT2 with an IC5050 value of >1 µM. The results suggest that KLISYRI has no clinically meaningful effect on the PK of drugs mediated by MDR1, BCRP, MRP2, BSEP, MATE1, MATE2-K, OAT1, OTA3, OATP1B1, OATP1B3, OCT1 and OCT2.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to evaluate the potential of tirbanibulin to induce carcinogenesis.
Tirbanibulin was negative in an in vitro bacterial reverse mutation (Ames) assay. Tirbanibulin was positive in an in vitro chromosomal aberration assay with Chinese hamster ovary (CHO) cells, an in vitro mouse lymphoma assay with L5178/TK+/- cells, and an in vivo micronucleus assay in rats.
Tirbanibulin was assessed for effects on fertility or reproductive function in rats. Reproductive performance of rats was unaffected by oral doses of tirbanibulin up to 4 mg/kg/day (24 times the MRHD on an AUC comparison basis) in males and 1 mg/kg/day (15 times the MRHD on an AUC comparison basis) in females. However, oral administration of 4 mg/kg/day of tirbanibulin to male rats adversely affected spermatogenesis, including reduced sperm count and motility, and increased observations of morphologically abnormal sperm. No effects on sperm were observed in males treated at 2 mg/kg/day (12 times the MRHD on an AUC comparison basis).
-
14 CLINICAL STUDIES
Actinic Keratosis of the Face or Scalp
Two double-blind, vehicle-controlled clinical trials (NCT03285477 and NCT03285490) were conducted with 702 adult subjects with actinic keratosis on the face or scalp. Subjects were randomized 1:1 to KLISYRI or vehicle. Subjects enrolled had 4 to 8 clinically typical, visible, and discrete AK lesions in a contiguous area of 25 cm2 on the face or scalp. Subjects had an average age of 70 years (range 45 to 96 years), were predominantly White (99%), male (87%), with Fitzpatrick skin types I or II (72%) and actinic keratosis on the face (68%) or scalp (32%). Treatment groups were comparable across all demographics and baseline characteristics, including AK lesion count and distribution on the face or scalp.
Subjects received 5 consecutive days of once daily treatment with either KLISYRI (353) or vehicle control (349) to the treatment field. Subjects with complete (100%) clearance of AK lesions in the treatment area at Day 57 returned to the clinic for recurrence assessment every 3 months for a total of 12 months post-Day 57.
The primary efficacy endpoint was complete (100%) clearance of AK lesions in the treatment area, defined as the proportion of subjects at Day 57 with no clinically visible AK lesions in the treatment area and the secondary endpoint was partial (≥75%) clearance of AK lesions in the treatment area. Results from both trials are presented below.
Table 3 Complete (100%) AK Clearance Rates on Day 57 in Adults with AK on the Face or Scalp for the Two Phase 3 Trials (Intent to Treat [ITT] Population) a. Based on Mantel-Haenszel method
Study 1 Study 2 KLISYRI
N = 175
n/N (%)Vehicle
N = 176
n/N (%)Treatment difference (KLISYRI-Vehicle) 95% Confidence Interval for the Treatment difference KLISYRI
N = 178
n/N (%)Vehicle
N = 173
n/N (%)Treatment difference (KLISYRI-Vehicle) 95% Confidence Interval for the Treatment difference All subjects 77/175
(44%)8/176
(5%)40%a (31.6%, 47.5%)a 97/178
(54%)22/173
(13%)42%a (33.1%, 50.7%)a Face 60/119
(50%)7/121
(6%)45% -- 73/119
(61%)16/118
(14%)48% -- Scalp 17/56
(30%)1/55
(2%)29% -- 24/59
(41%)6/55
(11%)30% -- Table 4 Partial (≥ 75%) AK Clearance Rates on Day 57 in Adults with AK on the Face or Scalp for the Two Phase 3 Trials (Intent to Treat [ITT] Population) a. Based on Mantel-Haenszel method
Study 1 Study 2 KLISYRI
N = 175
n/N (%)Vehicle
N = 176
n/N (%)Treatment difference (KLISYRI-Vehicle) 95% Confidence Interval for the Treatment difference KLISYRI
N = 178
n/N (%)Vehicle
N = 173
n/N (%)Treatment difference (KLISYRI-Vehicle) 95% Confidence Interval for the Treatment difference All subjects 119/175
(68%)29/176
(16%)52%a (42.9%, 60.3%)a 136/178
(76%)34/173
(20%)57% a (48.3%, 65.4%)a Face 90/119
(76%)23/121
(19%)57% -- 95/119 (80%) 26/118
(22%)58% -- Scalp 29/56
(52%)6/55
(11%)41% -- 41/59
(69%)8/55
(15%)55% -- Efficacy was consistent across sex and age (<65 and ≥65 years) subgroups.
Subjects who achieved 100% clearance of AK lesions in the treatment area at Day 57 continued to be followed for up to 12 months following Day 57 to determine the recurrence rate. Recurrence was defined as the proportion of subjects with any identified AK lesion (new or previous lesion) in the previously treated area who achieved 100% clearance at Day 57. Of the 174 subjects treated with KLISYRI who were followed, the recurrence rate at 12 months post-Day 57 was 73%.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
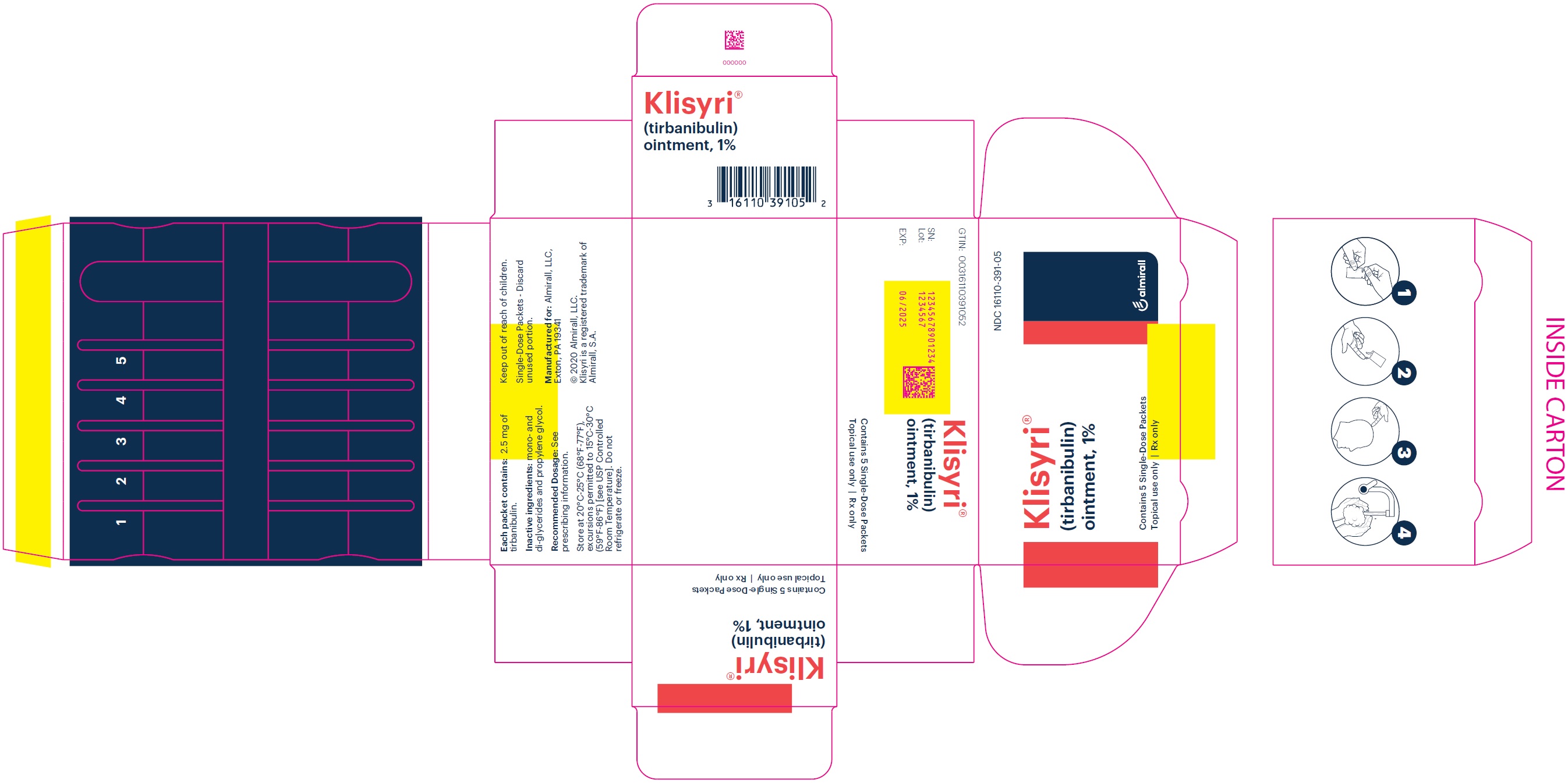
KLISYRI is a white to off-white ointment and is supplied in unit-dose packets containing either 250 mg or 350 mg of tirbanibulin ointment 1%. Discard each unit-dose packet after use.
NDC: 16110-391-05 (5 unit-dose packets each containing 250 mg of ointment)
NDC: 16110-391-55 (5 unit-dose packets each containing 350 mg of ointment) -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Ophthalmic Adverse Reactions
Advise patients that KLISYRI is not for ophthalmic use. Advise patients to avoid application around the eyes, and transfer of the drug into the eyes and to the periocular area. If accidental exposure occurs, advise patients to flush eyes with water and seek medical care [see Warnings and Precautions (5.1)].
Local Skin Reactions
Inform patients that treatment with KLISYRI may lead to local skin reactions [see Warnings and Precautions (5.2)].
Important Administration Instructions
Advise patients that KLISYRI is for topical use only. Advise patients to avoid application near and around the eyes, mouth and lips.
Instruct patients to:
- Wash hands well after applying KLISYRI to avoid transfer of the drug into the eyes and to the periocular area after application.
- Avoid washing and touching the treated area for 8 hours after treatment. Following this time, patients may wash the area with a mild soap and water [see Dosage and Administration (2)].
- Avoid inadvertent transfer of KLISYRI to other areas, or to another person.
Manufactured for: Almirall, LLC
Malvern, PA. 19355, USA -
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 08/2021
Patient Information
KLISYRI (klye si' ree)
(tirbanibulin)
ointmentImportant: KLISYRI is for use on the skin only (topical). Do not use KLISYRI in, around, or near your eyes, mouth or lips.
What is KLISYRI?
KLISYRI is a prescription medicine used on the skin to treat actinic keratosis on the face or scalp.
It is not known if KLISYRI is safe and effective in children less than 18 years of age.
Before using KLISYRI, tell your healthcare provider about all of your medical conditions, including if you:
- are being treated or have been treated for actinic keratosis with any other medicine, procedure, or surgery. You should not use KLISYRI until your skin has healed from other treatments.
- have other skin problems in the treatment area.
- are pregnant or plan to become pregnant. It is not known if KLISYRI can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if KLISYRI passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with KLISYRI.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I use KLISYRI?
- Use KLISYRI as your healthcare provider tells you to. KLISYRI is for skin use only.
- Apply KLISYRI to evenly cover the treatment area(s) on the face or scalp 1 time a day for 5 days in a row (consecutive) using 1 single dose packet with each application. Do not apply KLISYRI to other areas.
- Do not use more KLISYRI than you need to cover the treatment area. Using too much KLISYRI, or using it too often, or for too long can increase your chances of having a severe skin reaction or other side effects.
- Do not cover the treatment area with a dressing after application of KLISYRI. Irritation of the skin may happen if a dressing is applied to the treatment area.
-
Do not get KLISYRI in, around, or near your eyes. Do not touch your eyes while you are applying KLISYRI.
- Wash your hands right away with water and soap after applying KLISYRI. After applying KLISYRI, be careful to keep KLISYRI on the treated area from coming into contact with your eyes. Irritation may happen if you get KLISYRI in your eyes.
- If you accidently get KLISYRI in your eyes, flush them with water and get medical care as soon as possible. See, “What are the possible side effects of KLISYRI?”
- Do not get KLISYRI in, around, or near your mouth or lips.
- Avoid washing and touching the treated area for approximately 8 hours after application of KLISYRI. After 8 hours, you may wash the area with a mild soap and water.
- Avoid transferring the product to other areas after application.
- Throw away any open packet of KLISYRI after use even if there is medicine still left in it.
What are the possible side effects of KLISYRI?
KLISYRI may cause serious side effects, including:
- Eye irritation can happen if KLISYRI gets into your eyes. If you accidently get KLISYRI into your eyes, flush them with water and get medical care as soon as possible.
- Local skin reactions are common but can also be severe during treatment with KLISYRI. Call your healthcare provider if you develop any local skin reactions including redness, flaking or scaling, crusting, or swelling that is more severe, or if you get blisters, peeling, pus, ulcers, or breakdown of your skin.
The most common side effects of KLISYRI include: itching or pain in the treatment area.
These are not all of the possible side effects of KLISYRI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store KLISYRI?
- Store at room temperature between 68°F to 77°F (20°C to 25°C) .
- Do not refrigerate or freeze.
- Safely throw away used KLISYRI packets in household trash.
- Keep KLISYRI and all medicines out of the reach of children.
General Information about the safe and effective use of KLISYRI.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KLISYRI for a condition for which it was not prescribed. Do not give KLISYRI to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about KLISYRI that is written for health professionals.
What are the ingredients of KLISYRI?
Active ingredient: Tirbanibulin
Inactive ingredients: Mono- and di-glycerides and propylene glycol.
Manufactured for: Almirall, LLC, Malvern, PA. 19355, USA.
For more information, call 1-800-KLISYRI or visit www.KLISYRI.com.
- PRINCIPAL DISPLAY PANEL - NDC: 16110-391-05 - Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 16110-391-55 - Carton Label
-
INGREDIENTS AND APPEARANCE
KLISYRI
tirbanibulin ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 16110-391 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TIRBANIBULIN (UNII: 4V9848RS5G) (TIRBANIBULIN - UNII:4V9848RS5G) TIRBANIBULIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONO AND DIPALMITOSTEARATE (UNII: KC98RO82HJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 16110-391-05 5 in 1 CARTON 12/14/2020 1 0.25 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 16110-391-55 5 in 1 CARTON 06/07/2024 2 0.35 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213189 12/14/2020 Labeler - Almirall, LLC (605425912)
Trademark Results [Klisyri]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KLISYRI 88165309 5849059 Live/Registered |
Almirall, S.A. 2018-10-23 |
 KLISYRI 79302906 not registered Live/Pending |
Almirall, S.A. 2020-09-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.