MULTIHANCE- gadobenate dimeglumine injection, solution
MultiHance by
Drug Labeling and Warnings
MultiHance by is a Prescription medication manufactured, distributed, or labeled by BRACCO DIAGNOSTICS INC, BRACCO IMAGING SPA, BIPSO GmbH, Labor LS SE & Co. KG, PATHEON ITALIA SPA, BioChem Labor für biologishe und chemische Analytik GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MULTIHANCE safely and effectively. See full prescribing information for MULTIHANCE.
MultiHance (gadobenate dimeglumine) Injection
Initial U.S. Approval: 2004WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
See full prescribing information for complete boxed warning
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
-
The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
- magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and pediatric patients (including term neonates), to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine, and associated tissues. (1.1)
- magnetic resonance angiography (MRA) to evaluate adults with known or suspected renal or aorto-ilio-femoral occlusive vascular disease. (1.2)
DOSAGE AND ADMINISTRATION
- The recommended dose of MultiHance is 0.2 mL/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection.
- For MRI of the CNS in pediatric patients below 2 years of age the recommended dosage range is 0.1 to 0.2 mL/kg.
- To ensure complete injection of the contrast medium, follow the injection with a saline flush of at least 5 mL in MRI of the CNS and at least 20 mL in MRA. (2)
DOSAGE FORMS AND STRENGTHS
Each mL of MultiHance Injection contains 529 mg gadobenate dimeglumine and is available in single use vials. (3)
CONTRAINDICATIONS
MultiHance is contraindicated in patients with known allergic or hypersensitivity reactions to gadolinium-based contrast agents. (4)
WARNINGS AND PRECAUTIONS
- Nephrogenic Systemic Fibrosis has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeated dosing appears to increase the risk. (5.1)
- Hypersensitivity: anaphylactic/anaphylactoid reactions with cardiovascular, respiratory and cutaneous manifestations, ranging from mild to severe reactions including shock can occur. Monitor patients closely for need of emergency cardiorespiratory support. (5.2)
- Gadolinium is retained for months or years in brain, bone, and other organs. (5.3)
ADVERSE REACTIONS
The most commonly reported adverse reactions are nausea (1.3%) and headache (1.2%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc. at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2018
-
The risk for NSF appears highest among patients with:
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
1 INDICATIONS AND USAGE
1.1 Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
1.2 Magnetic Resonance Angiography (MRA) of Renal and Aorto-ilio-femoral Vessels
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Imaging Instructions
2.2 Dosing Table
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Nephrogenic Systemic Fibrosis (NSF)
5.2 Hypersensitivity Reactions
5.3 Gadolinium Retention
5.4 Acute Renal Failure
5.5 Extravasation and Injection Site Reactions
5.6 Cardiac Arrhythmias
5.7 Interference with Visualization of Certain Lesions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
7.1 Transporter-Based Drug-Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 MRI of the CNS
14.2 MRA of Renal and Aorto-ilio-femoral Vessels
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs.
-
The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration. [see Warnings and Precautions (5.1)]
-
The risk for NSF appears highest among patients with:
-
1 INDICATIONS AND USAGE
1.1 Magnetic Resonance Imaging (MRI) of the Central Nervous System (CNS)
MultiHance is indicated for intravenous use in magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and pediatric patients (including term neonates), to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine, and associated tissues.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Imaging Instructions
2.1.1 MRI of the CNS
In adults and in pediatric patients over 2 years of age, the recommended dose of MultiHance for MRI of the CNS is 0.2 mL/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection. In pediatric patients below 2 years of age, the recommended dosage range is 0.1 to 0.2 mL/kg administered as a rapid bolus intravenous injection. To ensure complete injection of the contrast medium, follow the injection with a saline flush of at least 5 mL. Imaging of the CNS can be performed starting immediately after the bolus injection of MultiHance.
2.1.2 MRA of Renal and Aorto-ilio-femoral Vessels
For MRA examination, the recommended dose is 0.2 mL/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection followed by at least 20 mL saline flush either manually or using an automatic injector system. Start imaging immediately after the administration of MultiHance, with scan delay calculated by test bolus or automatic bolus detection technique. If an automatic contrast detection pulse sequence is not used for bolus timing, then a test bolus injection of 1-2 mL of MultiHance should be used to calculate the appropriate scan delay.
2.2 Dosing Table
*For pediatric patients less than 2 years of age, one-half of the per kg dose may be used. TABLE 1: WEIGHT-BASED DOSING VOLUMES FOR:
CNS IMAGING (ADULTS AND PEDIATRICS ≥2 YEARS OF AGE*)
AND
MRA IMAGING (ADULTS ONLY)0.1mM/kg dose Kilograms (Kg) Pounds (lb) Volume, Milliliters (mL) 2.5 5.5 0.5 5 11 1.0 10 22 2.0 15 33 3.0 20 44 4.0 25 55 5.0 30 66 6.0 35 77 7.0 40 88 8.0 45 99 9.0 50 110 10.0 55 121 11.0 60 132 12.0 65 143 13.0 70 154 14.0 75 165 15.0 80 176 16.0 85 187 17.0 90 198 18.0 95 209 19.0 100 220 20.0 105 231 21.0 110 242 22.0 115 253 23.0 120 264 24.0 125 275 25.0 130 286 26.0 135 297 27.0 140 308 28.0 145 319 29.0 150 330 30.0 2.3 Administration
Inspect the MultiHance vial visually for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or particulate matter is present. Draw MultiHance into a syringe and inject using sterile technique.
Do not mix intravenous medications or parenteral nutrition solutions with MultiHance. Do not administer other medications in the same intravenous line with MultiHance.
MultiHance vials are intended for single use only. Administer immediately after opening and discard any unused product.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
MultiHance is contraindicated in patients with known allergic or hypersensitivity reactions to gadolinium-based contrast agents [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Nephrogenic Systemic Fibrosis (NSF)
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following MultiHance administration to Bracco Diagnostics (1-800-257-5181) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age >60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination. The usefulness of hemodialysis in the prevention of NSF is unknown [see Dosage and Administration (2) and Clinical Pharmacology (12)].
5.2 Hypersensitivity Reactions
Anaphylactic and anaphylactoid reactions have been reported, involving cardiovascular, respiratory, and/or cutaneous manifestations. Some patients experienced circulatory collapse and died. In most cases, initial symptoms occurred within minutes of MultiHance administration and resolved with prompt emergency treatment.
Prior to MultiHance administration, ensure the availability of personnel trained and medications to treat hypersensitivity reactions. If such a reaction occurs stop MultiHance and immediately begin appropriate therapy. Additionally, consider the risk for hypersensitivity reactions, especially in patients with a history of hypersensitivity reactions or a history of asthma or other allergic disorders. Observe patients for signs and symptoms of a hypersensitivity reaction during and for up to 2 hours after MultiHance administration.
5.3 Gadolinium Retention
Gadolinium is retained for months or years in several organs. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (e.g. brain, skin, kidney, liver, and spleen). The duration of retention also varies by tissue and is longest in bone. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, gadolinium retention varies among the linear agents with Omniscan (gadodiamide) and Optimark (gadoversetamide) causing greater retention than other linear agents [Eovist (gadoxetate disodium), Magnevist (gadopentetate dimeglumine), MultiHance (gadobenate dimeglumine)]. Retention is lowest and similar among the macrocyclic GBCAs [Dotarem (gadoterate meglumine), Gadavist (gadobutrol), ProHance (gadoteridol)].
Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function [see Warnings and Precautions (5.1)]. There are rare reports of pathologic skin changes in patients with normal renal function. Adverse events involving multiple organ systems have been reported in patients with normal renal function without an established causal link to gadolinium retention [see Adverse Reactions (6.2)].
While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies, particularly closely spaced studies when possible.
5.4 Acute Renal Failure
In patients with renal insufficiency, acute renal failure requiring dialysis or worsening renal function have occurred with the use of gadolinium-based contrast agents. The risk of renal failure may increase with increasing dose of the contrast agent. Screen all patients for renal dysfunction by obtaining a history and/or laboratory tests. Consider follow-up renal function assessments for patients with a history of renal dysfunction.
5.5 Extravasation and Injection Site Reactions
Extravasation of MultiHance may lead to injection site reactions, characterized by local pain or burning sensation, swelling, blistering, and necrosis. In animal experiments, local reactions including eschar and necrosis were noted even on Day 8 post perivenous injection of MultiHance. Exercise caution to avoid local extravasation during intravenous administration of MultiHance. If extravasation occurs, evaluate and treat as necessary if local reactions develop.
5.6 Cardiac Arrhythmias
Cardiac arrhythmias have been observed in patients receiving MultiHance in clinical trials [see Adverse Reactions (6.1)]. Assess patients for underlying conditions or medications that predispose to arrhythmias.
A double-blind, placebo-controlled, 24-hour post dose continuous monitoring, crossover study in 47 subjects evaluated the effect of 0.2 mmol/kg MultiHance on ECG intervals, including QTc. The average changes in QTc values compared with placebo were minimal (<5 msec). QTc prolongation between 30 and 60 msec were noted in 20 subjects who received MultiHance vs. 11 subjects who received placebo. Prolongations ≥61 msec were noted in 6 subjects who received MultiHance and in 3 subjects who received placebo. None of these subjects had associated malignant arrhythmias. The effects on QTc by MultiHance dose, other drugs, and medical conditions were not systematically studied.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Nephrogenic systemic fibrosis [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials with MultiHance, a total of 4967 adult subjects (137 healthy volunteers and 4830 patients) received MultiHance at doses ranging from 0.005 to 0.4 mmol/kg. There were 2838 (57%) men and 2129 (43%) women with a mean age of 56.5 years (range 18 to 93 years). A total of 4403 (89%) subjects were Caucasian, 134 (3%) Black, 275 (6%) Asian, 40 (1%) Hispanic, 70 (1%) in other racial groups, and for 45 (1%) subjects, race was not reported.
The most commonly reported adverse reactions in adult subjects who received MultiHance were nausea (1.3%) and headache (1.2%). Most adverse reactions were mild to moderate in intensity. One subject experienced a serious anaphylactoid reaction with laryngeal spasm and dyspnea [see Warnings and Precautions (5.2)]. Serious adverse reactions consisting of convulsions, pulmonary edema, acute necrotizing pancreatitis, and anaphylactoid reactions were reported in 0.1% of subjects in clinical trials.
Adverse reactions that occurred in at least 0.5% of 4967 adult subjects who received MultiHance are listed below (Table 2), in decreasing order of occurrence within each system.
TABLE 2: ADVERSE REACTIONS REPORTED IN ≥ 0.5% OF ADULT SUBJECTS WHO RECEIVED MULTIHANCE IN CLINICAL TRIALS Number of subjects dosed 4967 Number of subjects with any adverse reaction 517 (10.4%) Gastrointestinal Disorders
Nausea67 (1.3%) General Disorders and Administration Site Disorders
Injection Site Reaction
Feeling Hot54 (1.1%)
49 (1.0%)Nervous System Disorders
Headache
Dysgeusia
Paresthesia
Dizziness60 (1.2%)
33 (0.7%)
24 (0.5%)
24 (0.5%)The following adverse reactions occurred in less than 0.5% of the 4967 adult subjects who received MultiHance. Serious adverse reactions described above are not repeated below.
Blood and Lymphatic System Disorders: Basophilia;
Cardiac Disorders: Atrioventricular block first degree;
Eye Disorders: Eye pruritus, eye swelling, ocular hyperemia, visual disturbance;
Gastrointestinal Disorders: Abdominal pain or discomfort, diarrhea, dry mouth, lip swelling, paraesthesia oral, tongue edema, vomiting;
General Disorders and Administration Site Conditions: Chest pain or discomfort, chills, malaise;
Immune System Disorders: Hypersensitivity;
Investigations: Nonspecific changes in laboratory tests (including hematology, blood chemistry, liver enzymes and urinalysis), blood pressure and electrocardiogram parameters (including PR, QRS and QT intervals and ST-T segment changes).
Musculoskeletal and Connective Tissue Disorders: Myalgia;
Nervous System Disorders: Parosmia, tremor;
Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, laryngospasm, nasal congestion, sneezing, wheezing;
Skin and Subcutaneous Tissue Disorders: Hyperhidrosis, pruritus, rash, swelling face, urticaria.In clinical trials of MultiHance in MRI of the CNS, 307 pediatric subjects received MultiHance at a dose of 0.1 mmol/kg. A total of 160 (52%) subjects were male and the overall mean age was 6.0 years (range, 2 days to 17 years). A total of 211 (69%) subjects were Caucasian, 24 (8%) Black, 15 (5%) Asian, 39 (13%), Hispanic, 2 (<1%) in other racial groups, and for 16 (5%), race was not reported.
Adverse reactions were reported for 14 (4.6%) of the subjects. The frequency and the nature of the adverse reactions were similar to those seen in the adult patients. The most commonly reported adverse reactions were vomiting (1.0%), pyrexia (0.7%), and hyperhidrosis (0.7%). No subject died during study participation.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post approval use of MultiHance. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Anaphylactic, anaphylactoid and hypersensitivity reactions manifested with various degrees of severity up to anaphylactic shock, loss of consciousness and death. The reactions generally involved signs or symptoms of respiratory, cardiovascular, and/or mucocutaneous abnormalities.
General Disorders and Administration Site Conditions: Extravasation of MultiHance may lead to injection site reactions, characterized by local pain or burning sensation, swelling, blistering, and necrosis [see Warnings and Precautions (5.4)].
Adverse events with variable onset and duration have been reported after GBCA administration [see Warnings and Precautions (5.3)]. These include fatigue, asthenia, pain syndromes, and heterogeneous clusters of symptoms in the neurological, cutaneous, and musculoskeletal systems.
-
7 DRUG INTERACTIONS
7.1 Transporter-Based Drug-Drug Interactions
MultiHance and other drugs may compete for the canalicular multispecific organic anion transporter (MOAT also referred to as MRP2 or ABCC2). Therefore MultiHance may prolong the systemic exposure of drugs such as cisplatin, anthracyclines (e.g. doxorubicin, daunorubicin), vinca alkaloids (e.g. vincristine), methotrexate, etoposide, tamoxifen, and paclitaxel. In particular, consider the potential for prolonged drug exposure in patients with decreased MOAT activity (e.g. Dubin Johnson syndrome).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
GBCAs cross the placenta and result in fetal exposure and gadolinium retention. The human data on the association between GBCAs and adverse fetal outcomes are limited and inconclusive (see Data). In animal reproduction studies, gadobenate dimeglumine has been shown to be teratogenic in rabbits following repeated intravenous administration during organogenesis at doses up to 6 times the recommended human dose. There were no adverse developmental effects observed in rats with intravenous administration of gadobenate dimeglumine during organogenesis at doses up to three times the recommended human dose (see Data). Because of the potential risks of gadolinium to the fetus, use MultiHance only if imaging is essential and cannot be delayed.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and is 15 to 20%, respectively.
Data
Human Data
Contrast enhancement is visualized in the placenta and fetal tissues after maternal GBCA administration.Cohort studies and case reports on exposure to GBCAs during pregnancy have not reported a clear association between GBCAs and adverse effects in the exposed neonates. However, a retrospective cohort study, comparing pregnant women who had a GBCA MRI to pregnant women who did not have an MRI, reported a higher occurrence of stillbirths and neonatal deaths in the group receiving GBCA MRI. Limitations of this study include a lack of comparison with non-contrast MRI and lack of information about the maternal indication for MRI. Overall, these data preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of GBCAs in pregnancy.
Animal Data
Gadolinium Retention
GBCAs administered to pregnant non-human primates (0.1 mmol/kg on gestational days 85 and 135) result in measurable gadolinium concentration in the offspring in bone, brain, skin, liver, kidney, and spleen for at least 7 months. GBCAs administered to pregnant mice (2 mmol/kg daily on gestational days 16 through 19) result in measurable gadolinium concentrations in the pups in bone, brain, kidney, liver, blood, muscle, and spleen at one month postnatal age.
Reproductive Toxicology
Gadobenate dimeglumine has been shown to be teratogenic in rabbits when administered intravenously at 2 mmol/kg/day (6 times the recommended human dose based on body surface area) during organogenesis (day 6 to 18) inducing microphthalmia/small eye and/or focal retinal fold in 3 fetuses from 3 separate litters. In addition, MultiHance administered intravenously at 3 mmol/kg/day (10 times the recommended human dose based on body surface area) has been shown to increase intrauterine deaths in rabbits. There was no evidence that MultiHance induced teratogenic effects in rats at doses up to 2 mmol/kg/day (3 times the recommended human dose based on body surface area), however, rat dams exhibited no systemic toxicity at this dose. There were no adverse effects on the birth, survival, growth, development and fertility of the F1 generation at doses up to 2 mmol/kg in a rat peri- and post-natal (Segment III) study.8.2 Lactation
Risk Summary
Limited literature reports that breastfeeding after gadobenate dimeglumine administration to the mother would result in the infant receiving an oral dose of 0.001%-0.04% of the maternal dose. There is no information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. Additionally, there is limited GBCA gastrointestinal absorption. The developmental and health benefits of breastfeeding should be considered together with the mother’s clinical need for MultiHance and any potential adverse effects on the breastfed infant from MultiHance or from the underlying maternal condition.8.4 Pediatric Use
MultiHance is approved for intravenous use for MRI of the CNS to visualize lesions with abnormal blood brain barrier or abnormal vascularity of the brain, spine, and associated tissues in pediatric patients from birth, including term neonates, to less than 17 years of age. Pediatric use is based on evidence of effectiveness in adults and in 202 pediatric patients 2 years of age and older, in addition to experience in 105 pediatric patients birth to less than 2 years of age that supported extrapolation from adult data [see Clinical Studies (14)]. Adverse reactions in pediatric patients were similar to those reported in adults [see Adverse Reactions (6.1)]. No dose adjustment according to age is necessary in pediatric patients two years of age and older. For pediatric patients, less than 2 years of age, the recommended dosage range is 0.1 to 0.2 mL/kg [see Dosage and Administration (2.1), Pharmacokinetics (12.3)]. The safety of MultiHance has not been established in preterm neonates.
8.5 Geriatric Use
Of the total number of 4967 adult subjects in clinical studies of MultiHance, 33% were 65 or older. No overall differences in safety or effectiveness were observed between these elderly subjects and the younger subjects.
The drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to MultiHance may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function it may be useful to monitor renal function.
-
10 OVERDOSAGE
Clinical consequences of overdosage with MultiHance have not been reported. Treatment of an overdosage should be directed toward support of vital functions and prompt institution of symptomatic therapy. In a Phase 1 clinical study, doses up to 0.4 mmol/kg were administered to patients. MultiHance has been shown to be dialyzable [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
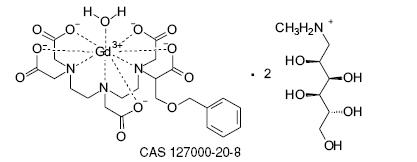
MultiHance injection is supplied as a sterile, nonpyrogenic, clear, colorless to slightly yellow aqueous solution intended for intravenous use only. Each mL of MultiHance contains 529 mg gadobenate dimeglumine and water for injection. MultiHance contains no preservatives.
Gadobenate dimeglumine is chemically designated as (4RS)-[4-carboxy-5,8,11-tris(carboxymethyl)- 1-phenyl-2-oxa-5,8,11-triazatridecan-13-oato(5-)] gadolinate(2-) dihydrogen compound with 1-deoxy-1-(methylamino)-D-glucitol (1:2) with a molecular weight of 1058.2 and an empirical formula of C22H28GdN3O11 2C7H17NO5. The structural formula is as follows:
MultiHance has a pH of 6.5-7.5. Pertinent physicochemical parameters are provided below:
Osmolality 1.970 osmol/kg @ 37°C Viscosity 5.3 mPas @ 37°C Density 1.220 g/mL @ 20°C MultiHance has an osmolality 6.9 times that of plasma (285 mOsmol/kg water) and is hypertonic under conditions of use.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gadobenate dimeglumine is a paramagnetic agent and, as such, develops a magnetic moment when placed in a magnetic field. The large magnetic moment produced by the paramagnetic agent results in a large local magnetic field, which can enhance the relaxation rates of water protons in its vicinity leading to an increase of signal intensity (brightness) of tissue.
In magnetic resonance imaging (MRI), visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occur with 1) differences in proton density; 2) differences of the spin-lattice or longitudinal relaxation times (T1); and 3) differences in the spin-spin or transverse relaxation time (T2). When placed in a magnetic field, gadobenate dimeglumine decreases the T1 and T2 relaxation time in target tissues. At recommended doses, the effect is observed with greatest sensitivity in the T1-weighted sequences.
12.2 Pharmacodynamics
Unlike other tested paramagnetic contrast agents (See Table 3), MultiHance demonstrates weak and transient interactions with serum proteins that causes slowing in the molecular tumbling dynamics, resulting in strong increases in relaxivity in solutions containing serum proteins. The improved relaxation effect can contribute to increased contrast-to-noise ratio and lesion-to-brain ratio, which may improve visualization.
TABLE 3: RELAXIVITY (mM–1s–1) OF GADOLINIUM CHELATES r1 and r2 relaxivities indicate the efficiency in shortening T1 and T2 relaxation times, respectively.
1 In heparinized human plasma, at 39°C.
2 In citrated human plasma, at 37°C.
-- Not availableHuman plasma r1 r2 Gadobenate 9.71 12.51 Gadopentetate 4.91 6.31 Gadodiamide 5.42 -- Gadoteridol 5.42 -- Disruption of the blood-brain barrier or abnormal vascularity allows enhancement by MultiHance of lesions such as neoplasms, abscesses, and infarcts. Uptake of MultiHance into hepatocytes has been demonstrated.
12.3 Pharmacokinetics
Three single-dose intravenous studies were conducted in 32 healthy male subjects to assess the pharmacokinetics of gadobenate dimeglumine. The doses administered in these studies ranged from 0.005 to 0.4 mmol/kg. Upon injection, the meglumine salt is completely dissociated from the gadobenate dimeglumine complex. Thus, the pharmacokinetics is based on the assay of gadobenate ion, the MRI contrast effective ion in gadobenate dimeglumine. Data for plasma concentration and area under the curve demonstrated linear dependence on the administered dose. The pharmacokinetics of gadobenate ion following intravenous administration can be best described using a two-compartment model.
Distribution
Gadobenate ion has a rapid distribution half-life (reported as mean ± SD) of 0.084 ± 0.012 to 0.605 ± 0.072 hours. Volume of distribution of the central compartment ranged from 0.074 ± 0.017 to 0.158 ± 0.038 L/kg, and estimates of volume of distribution by area ranged from 0.170 ± 0.016 to 0.282 ± 0.079 L/kg. These latter estimates are approximately equivalent to the average volume of extracellular body water in man. In vitro studies showed no appreciable binding of gadobenate ion to human serum proteins. Following GBCA administration, gadolinium is present for months or years in brain, bone, skin, and other organs [see Warnings and Precautions (5.3)].Elimination
Gadobenate ion is eliminated predominately via the kidneys, with 78% to 96% of an administered dose recovered in the urine. Total plasma clearance and renal clearance estimates of gadobenate ion were similar, ranging from 0.093 ± 0.010 to 0.133 ± 0.270 L/hr/kg and 0.082 ± 0.007 to 0.104 ± 0.039 L/hr/kg, respectively. The clearance is similar to that of substances that are subject to glomerular filtration. The mean elimination half-life ranged from 1.17 ± 0.26 to 2.02 ± 0.60 hours. A small percentage of the administered dose (0.6% to 4%) is eliminated via the biliary route and recovered in feces.Metabolism
There was no detectable biotransformation of gadobenate ion. Dissociation of gadobenate ion in vivo has been shown to be minimal, with less than 1% of the free chelating agent being recovered alone in feces.Pharmacokinetics in Special Populations
Renal Impairment: A single intravenous dose of 0.2 mmol/kg of MultiHance was administered to 20 subjects with impaired renal function (6 men and 3 women with moderate renal impairment [urine creatinine clearance >30 to <60 mL/min] and 5 men and 6 women with severe renal impairment [urine creatinine clearance >10 to <30 mL/min]). Mean estimates of the elimination half-life were 6.1 ± 3.0 and 9.5 ± 3.1 hours for the moderate and severe renal impairment groups, respectively as compared with 1.0 to 2.0 hours in healthy volunteers.
Hemodialysis: A single intravenous dose of 0.2 mmol/kg of MultiHance was administered to 11 subjects (5 males and 6 females) with end-stage renal disease requiring hemodialysis to determine the pharmacokinetics and dialyzability of gadobenate. Approximately 72% of the dose was recovered by hemodialysis over a 4-hour period. The mean elimination half-life on dialysis was 1.21 ± 0.29 hours as compared with 42.4 ± 24.4 hours when off dialysis.
Hepatic Impairment: A single intravenous dose of 0.1 mmol/kg of MultiHance was administered to 11 subjects (8 males and 3 females) with impaired liver function (Class B or C modified Child-Pugh Classification). Hepatic impairment had little effect on the pharmacokinetics of MultiHance with the parameters being similar to those calculated for healthy subjects.
Gender, Age, Race: A multiple regression analysis performed using pooled data from several pharmacokinetic studies found no significant effect of sex upon the pharmacokinetics of gadobenate. Clearance appeared to decrease slightly with increasing age. Since variations due to age appeared marginal, dosage adjustment for geriatric population is not recommended. Pharmacokinetic differences due to race have not been systematically studied.
Pediatric: A population pharmacokinetic analysis incorporated data from 25 healthy subjects (14 males and 11 females) and 15 subjects undergoing MR imaging of the central nervous system (7 males and 8 females) between ages of 2 and 16 years. The subjects received a single intravenous dose of 0.1 mmol/kg of MultiHance. The geometric mean Cmax was 62.3 μg/mL (n=16) in children 2 to 5 years of age, and 64.2 μg/mL (n=24) in children older than 5 years. The geometric mean AUC 0-∞ was 77.9 μg⋅h/mL in children 2-5 years of age (n=16) and 82.6 μg⋅h/mL in children older than 5 years (n=24). The geometric mean half-life was 1.2 hours in children 2 to 5 years of age and 0.93 hours in children older than 5 years. There was no significant gender-related difference in the pharmacokinetic parameters in the pediatric patients. Over 80% of the dose was recovered in urine after 24 hours. Pharmacokinetic simulations indicate similar AUC and Cmax values for MultiHance in pediatric subjects less than 2 years when compared to those reported for adults; no age-based dose adjustment is necessary for this pediatric population.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of MultiHance.
The results for MultiHance were negative in the following genetic toxicity studies: 1) in vitro bacteria reverse mutation assays, 2) an in vitro gene mutation assay in mammalian cells, 3) an in vitro chromosomal aberration assay, 4) an in vitro unscheduled DNA synthesis assay, and 5) an in vivo micronucleus assay in rats.
MultiHance had no effect on fertility and reproductive performance at IV doses of up to 2 mmol/kg/day (3 times the human dose on body surface basis) for 13 weeks in male rats and for 32 days in female rats. However, vacuolation in testes and abnormal spermatogenic cells were observed when MultiHance was intravenously administered to male rats at 3 mmol/kg/day (5 times the human dose on body surface basis) for 28 days. The effects were not reversible following 28-day recovery period. The effects were not reported in dog and monkey studies (at doses up to about 11 and 10 times the human dose on body surface basis for dogs (28 days dosing) and monkeys (14 days dosing), respectively).
-
14 CLINICAL STUDIES
14.1 MRI of the CNS
Adults:
MultiHance was evaluated in 426 adult patients in 2 controlled clinical trials of the central nervous system (Study A and Study B), enrolling 217 men and 209 women with a mean age of 52 years (range 18 to 88 years). The racial and ethnic representations were 88% Caucasian, 6% Black, 4% Hispanic, 1% Asian, and 1% other racial or ethnic groups. These trials were designed to compare MultiHance contrast MRI to non-contrast MRI alone. In Study A, patients highly suspected of having a lesion(s) of the CNS based on nuclear medicine imaging, computed tomography (CT), contrast CT, MRI, contrast- MRI, or angiography were randomized to receive two MRI evaluations with 0.05 mmol/kg (n=140) or 0.1 mmol/kg (n=136) of MultiHance. In Study B, patients with known metastatic disease to the CNS were randomized to receive two MRI evaluations with 0.05 mmol/kg (n=74) or 0.1 mmol/kg (n=76) of MultiHance. MRI scans were performed pre-contrast and within 5 minutes after each injection. The studies were designed to evaluate the effect of MultiHance MRI compared to the non-contrast MRI on a lesion level. Pre-contrast, post-contrast, and pre-plus-post contrast images (paired images) were independently evaluated by three blinded readers. The images were evaluated for the following endpoints using a scale from 0 to 4: the degree of lesion border delineation, the degree of visualization of lesion internal morphology, and the degree of lesion contrast enhancement. Lesion counting was also performed for the pre-contrast and paired image sets.The 0.1 mmol/kg dose of MultiHance demonstrated consistently better visualization for all readers for all visualization endpoints. However, the 0.05 mmol/kg dose of MultiHance provided inconsistent visualization results between readers.
Comparison of pre-contrast versus post-contrast (0.1 mmol/kg) images showed that the mean score differences were significant and favored contrast for subjects in Study B (all subjects with known metastatic lesions) and for subjects with known tumors in Study A. However, the mean score differences between the pre-contrast and post-contrast images were not significant for non-tumor patients in Study A. These negative results may be attributed to a lack of lesion enhancement in non-tumor CNS disease.
Table 4 shows a comparison of paired images (pre-and post-contrast) versus pre-contrast images with respect to the difference in the mean score and with respect to the proportion of lesions read as better, worse, or the same as the pre-contrast MRI images. Table 4 shows that based on a lesion-level analysis 0.1 mmol/kg MultiHance provided a statistically significant improvement for the three structural parameters evaluated. Also, more lesions were seen in the paired images than in the pre-contrast images alone.
(a) Difference of means = (paired mean) - (pre mean)
(b) Worse = paired score is less than the pre score
Same = paired score is the same as the pre score
Better = paired score is greater than the pre score
* Statistically significant for the mean (paired t test)Table 4: LESION LEVEL RESULTS OF MRI CENTRAL NERVOUS SYSTEM ADULT STUDIES WITH 0.1 mmol/kg MULTIHANCE Study A Study B Reader 1 Reader 2 Reader 3 Reader 1 Reader 2 Reader 3 Endpoints N = 395 N = 384 N = 299 N = 245 N = 275 N = 254 Border Delineation: Difference of Means (a) 0.8* 0.6* 0.8* 1.8* 1.5* 1.9* Worse (b)
Same
Better44 (11%)
146 (37%)
205 (52%)61 (16%)
168 (44%)
155 (40%)57 (19%)
89 (30%)
153 (51%)13 (5%)
11 (5%)
221 (90%)24 (9%)
19 (7%)
232 (84%)15 (6%)
18 (7%)
221 (87%)Internal Morphology: Difference of Means 0.8* 0.6* 0.7* 1.7* 1.4* 2.1* Worse
Same
Better37 (10%)
147 (37%)
211 (53%)63 (17%)
151 (39%)
170 (44%)62 (21%)
84 (28%)
153 (51%)13 (5%)
16 (7%)
216 (88%)26 (10%)
22 (8%)
227 (82%)14 (5%)
22 (9%)
218 (86%)Contrast Enhancement: Difference of Means 0.7* 0.5* 0.8* 1.9* 1.3* 1.9* Worse
Same
Better75 (19%)
148 (37%)
172 (44%)74 (19%)
152 (40%)
158 (41%)50 (17%)
109 (36%)
140 (47%)13 (5%)
11 (5%)
221 (90%)32 (12%)
21 (7%)
222 (81%)17 (7%)
14 (5%)
223 (88%)Pediatric 2 to 17 years
The efficacy and safety of MultiHance were evaluated in 92 pediatric patients with known or highly suspected disease of the central nervous system. MRI scans were performed pre-contrast and within 3 to 10 minutes following the administration of MultiHance 0.1 mmol/kg. Pre-contrast, post-contrast, and pre-plus-post contrast images (paired images) were independently evaluated by three blinded readers on a lesion level. The images were evaluated for the same endpoints as in the adult central nervous system trials using a scale from 0 to 4: the degree of lesion border delineation, the degree of visualization of lesion internal morphology, and the degree of lesion contrast enhancement. Lesion counting was also performed for the pre-contrast and paired image sets. The pre-contrast versus the paired image set was the primary comparison. Forty-nine percent of study subjects were male and the overall mean age was 10.6 years (range 2 to 17 years). The racial and ethnic representations were 77% Caucasian, 13% Asian, 5% Black, and 4% other racial or ethnic groups. MultiHance increased lesion border delineation, lesion internal morphology, and lesion contrast enhancement relative to non-contrast and these results were comparable to those seen in adults.Pediatrics below 2 years
A study of 90 pediatric patients younger than 2 years of age was performed which supports extrapolation of CNS efficacy findings from adults and older pediatric patients. Three independent, blinded readers evaluated pre-contrast MRI image sets and paired pre-plus-post-contrast MRI image sets using MultiHance and rated the images according to three co-primary endpoints at a lesion level for the primary analysis. Two of the three readers reported improvement in the paired image sets in each of the three co-primary endpoints of lesion border delineation, visualization of lesion internal morphology, and lesion contrast enhancement.14.2 MRA of Renal and Aorto-ilio-femoral Vessels
Safety and efficacy of MultiHance for use in MRA were evaluated in two prospective, multi-center, open-label, clinical trials (one for each arterial vascular territory: renal and aorto-ilio-femoral). Out of 580 patients who received Multihance in these two trials, 62.2% were men and 90.9% were Caucasian; the average age was 63.4 years (range 18 to 93 years). In both trials, patients with known or suspected arterial disease underwent MRA with and without MultiHance as well as catheter-based digital subtraction angiography (DSA). Assessment of diagnostic efficacy for detecting/excluding clinically significant steno-occlusive disease (≥ 51% stenosis measured with electronic calipers) was based on comparisons of sensitivity and specificity between MultiHance MRA and non-contrast MRA, with DSA as a reference standard.
In each vascular territory, the primary efficacy analyses were designed to demonstrate superiority in sensitivity and non-inferiority in specificity of MultiHance MRA to non-contrast MRA at the vessel-segment level. The interpretation of MRA images from both trials was conducted by three independent radiologist readers who were blinded to clinical data, including the DSA results. The pre-specified success criteria were to be achieved by at least the same two readers for all primary analyses.
Results of both trials showed a statistically significant increase in sensitivity and specificity of MultiHance MRA over non-contrast MRA in detecting clinically significant steno-occlusive disease.
Table 5 summarizes the efficacy results by reader.
TABLE 5. PERFORMANCE CHARACTERISTICS OF MULTIHANCE-MRA AND NON-CONTRAST MRA * (Based on General Delta Method) READER AORTO-ILIO-FEMORAL ARTERIES SENSITIVITY SPECIFICITY MultiHance
MRA [A]Non-contrast
MRA [B][A] – [B]
(95% CI)*MultiHance
MRA [A]Non-contrast
MRA [B][A] – [B]
(95% CI)*1 77.8% 73.7% 4.5
(1.5, 7.6)88.1% 78.5% 10.0
(7.3, 12.6)2 65.2% 52.5% 12.6
(8.5, 16.6)94.2% 89.4% 4.9
(2.7, 7.1)3 69.0% 59.1% 10.0
(6.1, 14.0)90.0% 75.3% 14.9
(12.1, 17.8)READER RENAL ARTERIES SENSITIVITY SPECIFICITY MultiHance
MRA [A]Non-contrast
MRA [B][A] – [B]
(95% CI)*MultiHance
MRA [A]Non-contrast
MRA [B][A] – [B]
(95% CI)*1 67.8% 47.0% 20.8
(12.8, 28.9)94.0% 86.1% 8.3
(4.2, 12.4)2 62.4% 46.7% 16.2
(6.8, 25.6)94.0% 83.5% 10.3
(5.5, 15.0)3 65.5% 39.6% 25.3
(15.9, 34.6)94.7% 87.3% 8.0
(3.6, 12.5) -
16 HOW SUPPLIED/STORAGE
AND HANDLING
16.1 How Supplied
MultiHance (gadobenate dimeglumine) is a clear, colorless to slightly yellow solution containing 529 mg gadobenate dimeglumine per mL. MultiHance is supplied in glass vials; each single dose vial is rubber stoppered with an aluminum seal and the contents are sterile. MultiHance is supplied in boxes of:Five 5 mL single dose 10 mL vials (NDC: 0270-5164-12)
Five 10 mL single dose 20 mL vials (NDC: 0270-5164-13)
Five 15 mL single dose 20 mL vials (NDC: 0270-5164-14)
Five 20 mL single dose 20 mL vials (NDC: 0270-5164-15)16.2 Storage and Handling
Store at 25°C (77°F), excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature]. Do not freeze. -
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Nephrogenic Systemic Fibrosis
Instruct patients to inform their physician if they:- have a history of kidney and/or liver disease, or
- have recently received a GBCA.
GBCAs increase the risk for NSF among patients with impaired elimination of the drugs. To counsel patients at risk for NSF:
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment.
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following MultiHance administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness.
Common Adverse Reactions
Inform patients that they may experience:- reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site
- side effects of feeling hot, nausea, and headache.
Gadolinium Retention
Advise patients that gadolinium is retained for months or years in brain, bone, skin, and other organs in patients with normal renal function. The clinical consequences of retention are unknown. Retention depends on multiple factors and is greater following administration of linear GBCAs than following administration of macrocyclic GBCAs [see Warnings and Precautions (5.3)].Rx only
US Patent No. 4,916,246Manufactured for
Bracco Diagnostics Inc.
Monroe Township, NJ 08831 -
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued 04/2018
COEB403MEDICATION GUIDE
MULTIHANCE®(məl-tē-han(t)s)
(gadobenate dimeglumine)
Injection for intravenous useWhat is MULTIHANCE? - MULTIHANCE is a prescription medicine called a gadolinium-based contrast agent (GBCA). MULTIHANCE, like other GBCAs, is injected into your vein and used with a magnetic resonance imaging (MRI) scanner.
- An MRI exam with a GBCA, including MULTIHANCE, helps your doctor to see problems better than an MRI exam without a GBCA.
- Your doctor has reviewed your medical records and has determined that you would benefit from using a GBCA with your MRI exam.
What is the most important information I should know about MULTIHANCE? - MULTIHANCE contains a metal called gadolinium. Small amounts of gadolinium can stay in your body including the brain, bones, skin and other parts of your body for a long time (several months to years).
- It is not known how gadolinium may affect you, but so far, studies have not found harmful effects in patients with normal kidneys.
- Rarely, patients have reported pains, tiredness, and skin, muscle or bone ailments for a long time, but these symptoms have not been directly linked to gadolinium.
- There are different GBCAs that can be used for your MRI exam. The amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after Omniscan or Optimark than after Eovist, Magnevist, or MultiHance. Gadolinium stays in the body the least after Dotarem, Gadavist, or ProHance.
- People who get many doses of gadolinium medicines, women who are pregnant and young children may be at increased risk from gadolinium staying in the body.
- Some people with kidney problems who get gadolinium medicines can develop a condition with severe thickening of the skin, muscles and other organs in the body (nephrogenic systemic fibrosis). Your healthcare provider should screen you to see how well your kidneys are working before you receive MULTIHANCE.
Do not receive MULTIHANCE if you have had a severe allergic reaction to GBCAs including gadobenate dimeglumine, or any of the ingredients in MULTIHANCE. Before receiving MULTIHANCE, tell your healthcare provider about all your medical conditions, including if you: - have had any MRI procedures in the past where you received a GBCA. Your healthcare provider may ask you for more information including the dates of these MRI procedures.
- are pregnant or plan to become pregnant. It is not known if MULTIHANCE can harm your unborn baby. Talk to your healthcare provider about the possible risks to an unborn baby if a GBCA such as MULTIHANCE is received during pregnancy
- have kidney problems, diabetes, or high blood pressure.
- have had an allergic reaction to dyes (contrast agents) including GBCAs
What are the possible side effects of MULTIHANCE? - See “What is the most important information I should know about MULTIHANCE?”
- Allergic reactions. MULTIHANCE can cause allergic reactions that can sometimes be serious. Your healthcare provider will monitor you closely for symptoms of an allergic reaction.
These are not all the possible side effects of MULTIHANCE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of MULTIHANCE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about MULTIHANCE that is written for health professionals.What are the ingredients in MULTIHANCE?
Active ingredient: gadobenate dimeglumine
Inactive ingredients: water
Manufactured by: BIPSO GmbH-78224 Singen (Germany)
Manufactured for: Bracco Diagnostics Inc., Monroe Township, NJ 08831
US Patent No. 4,916,246
For more information, go to www.imaging.bracco.com or call 1-800-257-5181. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MULTIHANCE
gadobenate dimeglumine injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0270-5164 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength gadobenate dimeglumine (UNII: 3Q6PPC19PO) (GADOLINIUM CATION (3+) - UNII:AZV954TZ9N) gadobenate dimeglumine 529 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0270-5164-12 5 in 1 BOX 11/23/2004 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 0270-5164-13 5 in 1 BOX 11/23/2004 2 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 3 NDC: 0270-5164-14 5 in 1 BOX 11/23/2004 3 15 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 4 NDC: 0270-5164-15 5 in 1 BOX 11/23/2004 4 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021357 11/23/2004 Labeler - BRACCO DIAGNOSTICS INC (849234661) Registrant - BRACCO DIAGNOSTICS INC (849234661) Establishment Name Address ID/FEI Business Operations BRACCO IMAGING SPA 434384007 API MANUFACTURE(0270-5164) Establishment Name Address ID/FEI Business Operations BIPSO GmbH 342104149 MANUFACTURE(0270-5164) Establishment Name Address ID/FEI Business Operations Takeda GmbH 313270016 ANALYSIS(0270-5164) Establishment Name Address ID/FEI Business Operations Labor LS SE & Co. KG 314929072 ANALYSIS(0270-5164) Establishment Name Address ID/FEI Business Operations Patheon Italia S.p.A 434078638 MANUFACTURE(0270-5164) , ANALYSIS(0270-5164)
Trademark Results [MultiHance]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MULTIHANCE 76615156 3061811 Live/Registered |
Bracco International B.V. 2004-10-08 |
 MULTIHANCE 75202806 2194677 Dead/Cancelled |
BRACCO INTERNATIONAL B.V. 1996-11-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.