NAGLAZYME- galsulfase solution
NAGLAZYME by
Drug Labeling and Warnings
NAGLAZYME by is a Prescription medication manufactured, distributed, or labeled by BioMarin Pharmaceutical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NAGLAZYME® safely and effectively. See full prescribing information for NAGLAZYME.
NAGLAZYME (galsulfase) injection, for intravenous use
Initial U.S. Approval: 2005INDICATIONS AND USAGE
NAGLAZYME is a hydrolytic lysosomal glycosaminoglycan (GAG)-specific enzyme indicated for patients with Mucopolysaccharidosis VI (MPS VI; Maroteaux-Lamy syndrome). NAGLAZYME has been shown to improve walking and stair-climbing capacity (1).
DOSAGE AND ADMINISTRATION
The recommended dosage is 1 mg per kg of body weight administered once weekly as an intravenous infusion (2).
DOSAGE FORMS AND STRENGTHS
Injection: 5 mg/5 mL (1 mg/mL) in a single-dose vial (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- Anaphylaxis and Hypersensitivity Reactions: Life-threatening anaphylactic reactions have been observed in some patients during NAGLAZYME infusions and up to 24 hours after infusion. If anaphylaxis or severe hypersensitivity reactions occur, immediately discontinue infusion and initiate appropriate treatment, which may include resuscitation, epinephrine, administering additional antihistamines, antipyretics or corticosteroids (5.1).
-
Immune-mediated Reactions: Immune-mediated reactions can occur with NAGLAZYME. Monitor patients for the development of immune complex-mediated reactions while receiving NAGLAZYME (5.2).
-
Risk of Acute Cardiorespiratory Failure: Caution should be exercised when administering NAGLAZYME to patients susceptible to fluid volume overload. Consider a decreased total infusion volume and infusion rate when administering NAGLAZYME to these patients. Appropriate medical monitoring and support measures should be available during infusion (2.1, 5.3).
-
Acute Respiratory Complications: Sleep apnea is common in MPS VI patients and antihistamine pretreatment may increase the risk of apneic episodes. Appropriate respiratory support should be available during infusion (5.4).
-
Infusion Reactions: Pretreatment with antihistamines with or without antipyretics is recommended prior to the start of infusion to reduce the risk of infusion-reactions. If infusion reactions occur, decreasing the infusion rate, temporarily stopping the infusion, or administering additional antihistamines and/or antipyretics is recommended (2.1, 5.5).
ADVERSE REACTIONS
The most common adverse reactions (≥10%) are: rash, pain, urticaria, pyrexia, pruritus, chills, headache, nausea, vomiting, abdominal pain and dyspnea. The most common adverse reactions requiring interventions are infusion-related reactions (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact: BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or go to www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Hypersensitivity Reactions
5.2 Immune-Mediated Reactions
5.3 Risk of Acute Cardiorespiratory Failure
5.4 Acute Respiratory Complications Associated with Administration
5.5 Infusion Reactions
5.6 Spinal or Cervical Cord Compression
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dosage regimen of NAGLAZYME is 1 mg per kg of body weight administered once weekly as an intravenous infusion.
Pretreatment with antihistamines with or without antipyretics is recommended 30 to 60 minutes prior to the start of the infusion [see Warnings and Precautions (5.5].
The total volume of the infusion should be delivered over a period of time of no less than 4 hours. NAGLAZYME should be diluted with 0.9% Sodium Chloride Injection, USP, to a final volume of 250 mL and delivered by controlled intravenous infusion using an infusion pump. The initial infusion rate should be 6 mL per hour for the first hour. If the infusion is well tolerated, the rate of infusion may be increased to 80 mL per hour for the remaining 3 hours. The infusion time can be extended up to 20 hours if infusion reactions occur.
For patients 20 kg and under or those who are susceptible to fluid volume overload, physicians may consider diluting NAGLAZYME in a volume of 100 mL [see Warnings and Precautions (5.3)]. The infusion rate (mL per hour) should be decreased so that the total infusion duration remains no less than 4 hours.
Each vial of NAGLAZYME provides 5 mg of galsulfase (expressed as protein content) in 5 mL of solution and is intended for single use only. Do not use the vial more than one time. The concentrated solution for infusion must be diluted with 0.9% Sodium Chloride Injection, USP, using aseptic techniques. Prepare NAGLAZYME using low-protein-binding containers and administer the diluted NAGLAZYME solution to patients using a low-protein-binding infusion set equipped with a low-protein-binding 0.2 µm in-line filter. There is no information on the compatibility of diluted NAGLAZYME with glass containers.
2.2 Instructions for Use
Prepare and use NAGLAZYME according to the following steps. Use aseptic techniques.
- Determine the number of vials to be used based on the patient's weight and the recommended dose of 1 mg per kg:
Patient's weight (kg) x 1 mL/kg of NAGLAZYME = Total number of mL of NAGLAZYME
Total number of mL of NAGLAZYME ÷ 5 mL per vial = Total number of vials
Round up to the next whole vial. Remove the required number of vials from the refrigerator to allow them to reach room temperature. Do not allow vials to remain at room temperature longer than 24 hours prior to dilution. Do not heat or microwave vials.
- Before withdrawing the NAGLAZYME solution from the vial, visually inspect each vial for particulate matter and discoloration. The NAGLAZYME solution should be clear to slightly opalescent and colorless to pale yellow. Some translucency may be present in the solution. Do not use if the solution is discolored or if there is particulate matter in the solution.
- From a 250 mL infusion bag of 0.9% Sodium Chloride Injection, USP, withdraw and discard a volume equal to the volume of NAGLAZYME solution to be added. If using a 100 mL infusion bag, this step is not necessary.
- Slowly withdraw the calculated volume of NAGLAZYME from the appropriate number of vials using caution to avoid excessive agitation. Do not use a filter needle, as this may cause agitation. Agitation may denature NAGLAZYME, rendering it biologically inactive.
- Slowly add the NAGLAZYME solution to the 0.9% Sodium Chloride Injection, USP, using care to avoid agitation of the solutions. Do not use a filter needle.
- Gently rotate the infusion bag to ensure proper distribution of NAGLAZYME. Do not shake the solution.
- Administer the diluted NAGLAZYME solution to patients using a low-protein-binding infusion set equipped with a low-protein-binding 0.2 µm in-line filter.
NAGLAZYME does not contain preservatives; therefore, after dilution with saline, the infusion bags should be used immediately. If immediate use is not possible, the diluted solution must be stored refrigerated at 2°C to 8°C (36°F to 46°F) and administered within 48 hours from the time of dilution to completion of administration. Other than during infusion, do not store the diluted NAGLAZYME solution at room temperature. Any unused product or waste material must be discarded and disposed of in accordance with local requirements.
NAGLAZYME must not be infused with other products in the infusion tubing. The compatibility of NAGLAZYME in solution with other products has not been evaluated.
- Determine the number of vials to be used based on the patient's weight and the recommended dose of 1 mg per kg:
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis and Hypersensitivity Reactions
Anaphylaxis and serious hypersensitivity reactions have been observed in patients during and up to 24 hours after NAGLAZYME infusion. Some of the reactions were life-threatening and included anaphylaxis, shock, respiratory distress, dyspnea, bronchospasm, laryngeal edema, and hypotension [see Adverse Reactions (6.1, 6.3)]. If anaphylaxis or other serious hypersensitivity reactions occur, NAGLAZYME should be immediately discontinued, and appropriate medical treatment should be initiated. In patients who have experienced anaphylaxis or other serious hypersensitivity reactions during infusion with NAGLAZYME, caution should be exercised upon rechallenge; appropriately trained personnel and equipment for emergency resuscitation (including epinephrine) should be available during infusion.
5.2 Immune-Mediated Reactions
Type III immune complex-mediated reactions, including membranous glomerulonephritis have been observed with NAGLAZYME, as with other enzyme replacement therapies. If immune-mediated reactions occur, discontinuation of the administration of NAGLAZYME should be considered, and appropriate medical treatment initiated. The risks and benefits of re-administering NAGLAZYME following an immune-mediated reaction should be considered. Some patients have successfully been rechallenged and have continued to receive NAGLAZYME under close clinical supervision [see Adverse Reactions (6.3)].
5.3 Risk of Acute Cardiorespiratory Failure
Caution should be exercised when administering NAGLAZYME to patients susceptible to fluid volume overload, such as patients weighing 20 kg or less, patients with acute underlying respiratory illness, or patients with compromised cardiac and/or respiratory function, because congestive heart failure may result. Appropriate medical support and monitoring measures should be readily available during NAGLAZYME infusion, and some patients may require prolonged observation times that should be based on the individual needs of the patient [see Adverse Reactions (6.3)].
5.4 Acute Respiratory Complications Associated with Administration
Sleep apnea is common in MPS VI patients and antihistamine pretreatment may increase the risk of apneic episodes. Evaluation of airway patency should be considered prior to initiation of treatment. Patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep should have these treatments readily available during infusion in the event of an infusion reaction, or extreme drowsiness/sleep induced by antihistamine use.
Consider delaying NAGLAZYME infusions in patients who present with an acute febrile or respiratory illness because of the possibility of acute respiratory compromise during infusion of NAGLAZYME.
5.5 Infusion Reactions
Because of the potential for infusion reactions, patients should receive antihistamines with or without antipyretics prior to infusion. Despite routine pretreatment with antihistamines, infusion reactions, some severe, occurred in 33 of 59 (56%) patients treated with NAGLAZYME. Serious adverse reactions during infusion included laryngeal edema, apnea, pyrexia, urticaria, respiratory distress, angioedema, and anaphylactoid reaction. Severe adverse reactions included urticaria, chest pain, rash, dyspnea, apnea, laryngeal edema, and conjunctivitis [see Adverse Reactions (6.1, 6.3)].
The most common symptoms of drug-related infusion reactions were pyrexia, chills, rash, urticaria, dyspnea, nausea, vomiting, pruritis, erythema, abdominal pain, hypertension, and headache. Respiratory distress, chest pain, hypotension, angioedema, conjunctivitis, tremor, and cough were also reported. Infusion reactions began as early as Week 1 and as late as Week 146 of NAGLAZYME treatment. Twenty-three of 33 patients (70%) experienced recurrent infusion reactions during multiple infusions though not always in consecutive weeks.
Symptoms typically abated with slowing or temporary interruption of the infusion and administration of additional antihistamines, antipyretics, and occasionally corticosteroids. Most patients were able to complete their infusions. Subsequent infusions were managed with a slower rate of NAGLAZYME administration, treatment with additional prophylactic antihistamines, and, in the event of a more severe reaction, treatment with prophylactic corticosteroids.
If severe infusion reactions occur, immediately discontinue the infusion of NAGLAZYME and initiate appropriate treatment. The risks and benefits of re-administering NAGLAZYME following a severe reaction should be considered.
No factors were identified that predisposed patients to infusion reactions. There was no association between severity of infusion reactions and titer of anti-galsulfase antibodies.
5.6 Spinal or Cervical Cord Compression
Spinal or cervical cord compression (SCC) with resultant myelopathy is a known and serious complication of MPS VI. SCC is expected to occur in the natural history of the disease, including in patients on NAGLAZYME. There have been post-marketing reports of patients treated with NAGLAZYME who experienced the onset or worsening of SCC requiring decompression surgery. Patients with MPS VI should be monitored for signs and symptoms of spinal/cervical cord compression (including back pain, paralysis of limbs below the level of compression, urinary and fecal incontinence) and given appropriate clinical care.
-
6 ADVERSE REACTIONS
Serious and or clinically significant adverse reactions described elsewhere in labeling include:
- Anaphylaxis and Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Immune-Mediated Reactions [see Warnings and Precautions (5.2)]
- Risk of Acute Cardiorespiratory Failure [see Warnings and Precautions (5.3)]
- Acute Respiratory Complications Associated with Administration [see Warnings and Precautions (5.4)]
- Infusion Reactions [see Warnings and Precautions (5.5)]
- Spinal or Cervical Cord Compression [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates observed in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
NAGLAZYME was studied in a randomized, double-blind, placebo-controlled trial in which 19 patients received weekly infusions of 1 mg/kg NAGLAZYME and 20 patients received placebo; of the 39 patients 66% were female, and 62% were White, non-Hispanic. Patients were aged 5 years to 29 years. NAGLAZYME-treated patients were approximately 3 years older than placebo-treated patients (mean age 13.7 years versus 10.7 years, respectively).
Serious adverse reactions experienced in this trial include apnea, pyrexia, and respiratory distress. Severe adverse reactions include chest pain, dyspnea, laryngeal edema, and conjunctivitis. The most common adverse reactions requiring interventions were infusion reactions.
Table 1 summarizes the adverse reactions that occurred in the placebo-controlled trial in at least 2 patients more in the NAGLAZYME‑treated group than in the placebo-treated group.
Table 1: Adverse Reactions that Occurred in the Placebo-Controlled Trial in at least 2 Patients More in the NAGLAZYME Group than in the Placebo Group MedDRA Preferred Term NAGLAZYME
(n = 19)Placebo
(n = 20*)No. Patients (%) No. Patients (%) - * One of the 20 patients in the placebo group dropped out after Week 4 infusion
All 19 (100) 20 (100) Abdominal Pain 9 (47) 7 (35) Ear Pain 8 (42) 4 (20) Arthralgia 8 (42) 5 (25) Pain 6 (32) 1 (5) Conjunctivitis 4 (21) 0 Dyspnea 4 (21) 2 (10) Rash 4 (21) 2 (10) Chills 4 (21) 0 Chest Pain 3 (16) 1 (5) Pharyngitis 2 (11) 0 Areflexia 2 (11) 0 Corneal Opacity 2 (11) 0 Gastroenteritis 2 (11) 0 Hypertension 2 (11) 0 Malaise 2 (11) 0 Nasal Congestion 2 (11) 0 Umbilical Hernia 2 (11) 0 Hearing Impairment 2 (11) 0 Four open-label clinical trials were conducted in MPS VI patients aged 3 months to 29 years with NAGLAZYME administered at doses of 0.2 mg/kg (n = 2), 1 mg/kg (n = 55), and 2 mg/kg (n = 2). The mean exposure to the recommended dose of NAGLAZYME (1 mg/kg) was 138 weeks (range = 54 to 261 weeks). Two infants (12.1 months and 12.7 months) were exposed to 2 mg/kg of NAGLAZYME for 105 and 81 weeks, respectively.
In addition to those listed in Table 1, common adverse reactions observed in the open-label trials include pruritus, urticaria, pyrexia, headache, nausea, and vomiting. The most common adverse reactions requiring interventions were infusion reactions. Serious adverse reactions included laryngeal edema, urticaria, angioedema, and other hypersensitivity reactions. Severe adverse reactions included urticaria, rash, and abdominal pain.
Observed adverse events in four open-label studies (up to 261 weeks treatment) were not different in nature or severity to those observed in the placebo-controlled study. No patients discontinued during open-label treatment with NAGLAZYME due to adverse events.
6.2 Immunogenicity
As with all the therapeutic proteins, there is potential for immunogenicity. The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibodies in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other galsulfase products may be misleading.
Ninety-eight percent (53/54) of patients treated with NAGLAZYME and evaluable for the presence of antibodies to galsulfase developed anti-galsulfase IgG antibodies within 4 to 8 weeks of treatment (in four clinical studies). In 19 patients treated with NAGLAZYME from the placebo-controlled study, serum samples were evaluated for a potential relationship of anti-galsulfase antibody development to clinical outcome measures. All 19 patients treated with NAGLAZYME developed antibodies specific to galsulfase; however, the analysis revealed no consistent predictive relationship between total antibody titer, neutralizing or IgE antibodies, and infusion‑associated reactions, urinary glycosaminoglycan (GAG) levels, or endurance measures. Antibodies were assessed for the ability to inhibit enzymatic activity but not cellular uptake.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post approval use of NAGLAZYME. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serious infusion reactions: anaphylaxis, shock, hypotension, bronchospasm, and respiratory failure [see Warnings and Precautions (5.1)].
Additional infusion reactions: pyrexia, erythema, pallor, bradycardia, tachycardia, hypoxia, cyanosis, tachypnea, and paresthesia.
During postmarketing surveillance, there has been a single case of membranous nephropathy and rare cases of thrombocytopenia reported. In the case of membranous nephropathy, renal biopsy revealed galsulfase‑immunoglobulin complexes in the glomeruli. With both membranous nephropathy and thrombocytopenia, patients have been successfully rechallenged and have continued to receive NAGLAZYME.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports and postmarketing experience with NAGLAZYME use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, galsulfase administered intravenously to pregnant rats and rabbits during the period of organogenesis, showed no evidence of harm to the fetus at doses of about 0.5 and 0.97 times, respectively for rats and rabbits, the recommended human dose of 1 mg/kg based on body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and embryo/fetal risk
Pregnancy can exacerbate preexisting clinical manifestations of MPS and lead to adverse pregnancy outcomes for both mother and fetus.
Data
Animal Data
Reproduction studies have been performed with intravenous galsulfase during the period of organogenesis in pregnant rats at doses of galsulfase up to 3 mg/kg/day (about 0.5 times the recommended human dose of 1 mg/kg based on the body surface area) and in pregnant rabbits at doses up to 3 mg/kg/day (about 0.97 times the recommended human dose of 1 mg/kg based on the body surface area) and have revealed no evidence of harm to the fetus due to galsulfase.
8.2 Lactation
Risk Summary
There are no data on the presence of galsulfase in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NAGLAZYME and any potential adverse effects on the breastfed infant from NAGLAZYME or from the underlying maternal condition.
8.4 Pediatric Use
Clinical studies with NAGLAZYME were conducted in 56 patients, ages 5 to 29 years, with the majority of these patients in the pediatric age group [see Clinical Studies (14)]. In addition, an open-label study was conducted in four infants (3 months to 12.7 months) treated with 1 mg/kg (n = 2) or 2 mg/kg (n = 2) of NAGLAZYME. Safety results in infants were consistent with results observed in patients 5 to 29 years old [see Adverse Reactions (6)].
-
11 DESCRIPTION
NAGLAZYME is a formulation of galsulfase, which is a purified human enzyme that is produced by recombinant DNA technology in a Chinese hamster ovary cell line. Galsulfase (glycosaminoglycan N–acetylgalactosamine 4-sulfatase, EC 3.1.6.12) is a lysosomal enzyme that catalyzes the cleavage of the sulfate ester from terminal N–acetylgalactosamine 4-sulfate residues of glycosaminoglycans (GAG), chondroitin 4-sulfate and dermatan sulfate.
Galsulfase is a glycoprotein with a molecular weight of approximately 56 kDa. The recombinant protein consists of 495 amino acids and possesses six asparagine‑linked glycosylation sites, four of which carry a bis‑mannose–6–phosphate residue for specific cellular recognition. Post-translational modification of Cys53 produces the catalytic amino acid residue, Cα-formylglycine, which is required for enzyme activity. NAGLAZYME has a specific activity of approximately 70 units per mg of protein content. One activity unit is defined as the amount of enzyme required to convert 1 micromole of 4-methylumbelliferyl sulfate to 4-methylumbelliferone and free sulfate per minute at 37°C.
NAGLAZYME is intended for intravenous infusion and is supplied as a sterile, nonpyrogenic, colorless to pale yellow, clear to slightly opalescent solution that must be diluted with 0.9% Sodium Chloride Injection, USP, prior to administration. NAGLAZYME is supplied in clear Type I glass 5 mL vials. Each vial provides 5 mg galsulfase, 43.8 mg sodium chloride, 6.20 mg sodium phosphate monobasic monohydrate, 1.34 mg sodium phosphate dibasic heptahydrate, and 0.25 mg polysorbate 80 in a 5 mL extractable solution with pH of approximately 5.8. NAGLAZYME does not contain preservatives. Each vial is for single use only.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mucopolysaccharide storage disorders are caused by the deficiency of specific lysosomal enzymes required for the catabolism of GAG. MPS VI is characterized by the absence or marked reduction in N–acetylgalactosamine 4-sulfatase. The sulfatase activity deficiency results in the accumulation of the GAG substrate, dermatan sulfate, throughout the body. This accumulation leads to widespread cellular, tissue, and organ dysfunction. NAGLAZYME is intended to provide an exogenous enzyme that will be taken up into lysosomes and increase the catabolism of GAG. Galsulfase uptake by cells into lysosomes is most likely mediated by the binding of mannose-6-phosphate-terminated oligosaccharide chains of galsulfase to specific mannose-6-phosphate receptors.
12.2 Pharmacodynamics
The responsiveness of urinary GAG to dosage alterations of NAGLAZYME is unknown, and the relationship of urinary GAG to other measures of clinical response has not been established. No association was observed between antibody development and urinary GAG levels [see Adverse Reactions (6.2)].
12.3 Pharmacokinetics
The pharmacokinetic parameters of galsulfase were evaluated in 13 patients with MPS VI who received 1 mg/kg of NAGLAZYME as a weekly 4-hour infusion for 24 weeks. The pharmacokinetic parameters at Week 1 and Week 24 are shown in Table 2.
Table 2: Pharmacokinetic Parameters (Median, Range) of Galsulfase in Patients with MPS VI - * Area under the plasma galsulfase concentration-time curve from start of infusion to 60 minutes post infusion.
Pharmacokinetic Parameter Week 1 Week 24 Cmax (mcg/mL) 0.8 (0.4 to 1.3) 1.5 (0.2 to 5.5) AUC0-t (hrmcg/mL)* 2.3 (1.0 to 3.5) 4.3 (0.3 to 14.2) Vz (mL/kg) 103 (56 to 323) 69 (59 to 2,799) CL (mL/kg/min) 7.2 (4.7 to 10.5) 3.7 (1.1 to 55.9) Half-life (min) 9 (6 to 21) 26 (8 to 40) Galsulfase pharmacokinetic parameters listed in Table 2 require cautious interpretation because of large assay variability. Development of anti-galsulfase antibodies appears to affect galsulfase pharmacokinetics, however, the data are limited.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with galsulfase.
Galsulfase at intravenous doses up to 3.0 mg/kg (about 0.5 times the recommended human dose of 1 mg/kg based on body surface area) was found to have no effect on the fertility and reproductive performance of male and female rats.
-
14 CLINICAL STUDIES
A total of 56 patients with MPS VI, ages 5 years to 29 years, were enrolled in four clinical studies. The majority of patients had severe manifestations of the disease as evidenced by poor performance on a test of physical endurance.
In the randomized, double-blind, multicenter, placebo-controlled clinical trial, 38 patients with MPS VI received 1 mg/kg NAGLAZYME or placebo, once-weekly for 24 weeks. The patients’ ages ranged from 5 to 29 years. Enrollment was restricted to patients with a 12‑minute walk distance of 5 to 400 meters. All patients were treated with antihistamines prior to each infusion.
The NAGLAZYME-treated group showed greater mean increases in the distance walked in 12 minutes (12‑minute walk test, 12‑MWT) and in the rate of stair climbing in a 3-minute stair climb test, compared with the placebo group (Table 3).
Table 3: Results from Placebo-Controlled Clinical Study - * One patient in the placebo group dropped out after 4 weeks of infusion
- † Observed mean of NAGLAZYME - Placebo ± SE
- ‡ Model-based mean of NAGLAZYME - Placebo ± SE, adjusted for baseline
- § p-value based on the model-based mean difference
NAGLAZYME Placebo NAGLAZYME
vs.
PlaceboBaseline Week 24 Change Baseline Week 24 Change Difference in
ChangesN 19 19 19 20 19* 19 Results from the 12-Minute Walk Test (Meters) Mean ± SD
Median
Percentiles
(25th, 75th)227 ± 170
210
90, 330336 ± 227
316
125, 483109 ± 154
48
7, 183381 ± 202
365
256, 560399 ± 217
373
204, 57326 ± 122
34
–3, 8983 ± 45†
92 ± 40‡
(p = 0.025)‡§Results from 3-Minute Stair Climb Test (Stairs/Minute) Mean ± SD
Median
Percentiles
(25th, 75th)19.4 ± 12.9
16.7
10.0, 26.326.9 ± 16.8
22.8
14.8, 33.07.4 ± 9.9
5.2
2.2, 9.931.0 ± 18.1
24.7
18.1, 51.532.6 ± 19.6
29.0
14.2, 57.92.7 ± 6.9
4.3
1.0, 6.24.7 ± 2.8†
5.7 ± 2.9‡
(p = 0.053)‡§Following the 24-week placebo-controlled study period, 38 patients received open-label NAGLAZYME for 72 weeks. Among the 19 patients who were initially randomized to NAGLAZYME and who continued to receive treatment for 72 weeks (total of 96 weeks), increases in the 12-MWT distance and in the rate of stair climbing were observed compared to the start of the open-label period (mean [ ± SD] change): 72 ± 116 meters and 5.6 ± 10.6 stairs/minute, respectively). Among the 19 patients who were randomized initially to placebo for 24 weeks, and then crossed over to treatment with NAGLAZYME, the increases after 72 weeks of NAGLAZYME treatment compared to the start of the open-label period, (mean [ ± SD] change): were 118 ± 127 meters and 11.1 ± 10.0 stairs/minute, for the 12-MWT and the rate of stair climbing, respectively.
Bioactivity was evaluated with urinary GAG concentration. Overall, 95% of patients showed at least a 50% reduction in urinary GAG levels after 72 weeks of treatment with NAGLAZYME. No patient receiving NAGLAZYME reached the normal range for urinary GAG levels [see Clinical Pharmacology (12.2)].
In an additional open-label extension study, patients receiving NAGLAZYME showed maintenance of initial improvement in endurance for approximately 240 weeks.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NAGLAZYME injection is supplied as a sterile colorless to pale yellow, clear to slightly opalescent solution in clear Type I glass 5 mL vials, containing 5 mg galsulfase (expressed as protein content) per 5 mL solution. The closure consists of a siliconized chlorobutyl rubber stopper and an aluminum seal with a plastic flip-off cap.
NDC: 68135-020-01, 5 mL vial
Store NAGLAZYME under refrigeration at 2°C to 8°C (36°F to 46°F). Do not freeze or shake. Protect from light. This product contains no preservatives.
-
17 PATIENT COUNSELING INFORMATION
Anaphylaxis, Hypersensitivity and Infusion Reactions
Inform the patient or caregiver that hypersensitivity reactions, including life-threatening anaphylaxis, and infusion reactions may occur with NAGLAZYME treatment. Advise the patient or caregiver to report immediately to a healthcare provider if signs or symptoms of a hypersensitivity or infusion reaction occur during infusion of NAGLAZYME. [see Warnings and Precautions (5.1, 5.5)].
Cardiac and Respiratory Adverse Reactions
Advise the patient or caregiver to report immediately to a healthcare provider if signs or symptoms of cardiac or respiratory decompensation occur during or following an infusion [see Warnings and Precautions (5.3, 5.4)] Inform patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep to have these treatments readily available during infusion or extreme drowsiness/sleep induced by antihistamine use.
NAGLAZYME is manufactured and distributed by:
BioMarin Pharmaceutical Inc.
Novato, CA 94949
US License Number 1649
1-866-906-6100 (phone)NAGLAZYME® is a registered trademark of BioMarin.
-
Packaging Components
NDC: 68135-020-01
Naglazyme®
(GALSULFASE)5 mg/5 mL
(1 mg/mL)Concentrated Solution For Intravenous Infusion Only
Must be diluted prior to use.
Rx Only
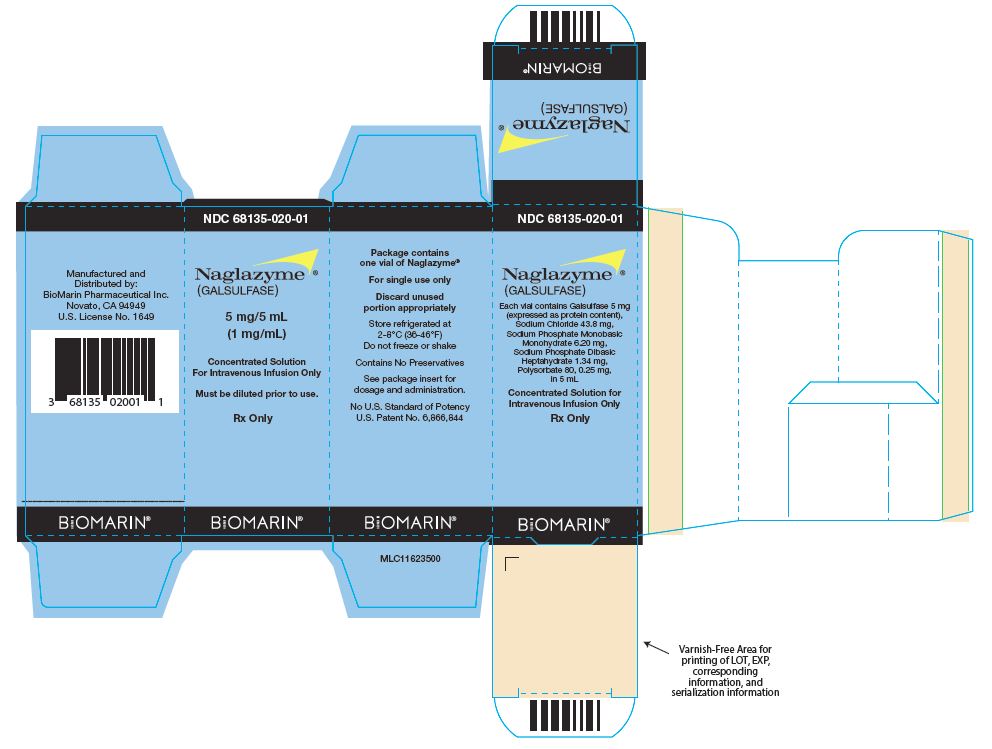
Naglazyme Carton
-
INGREDIENTS AND APPEARANCE
NAGLAZYME
galsulfase solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68135-020 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GALSULFASE (UNII: 59UA429E5G) (GALSULFASE - UNII:59UA429E5G) GALSULFASE 5 mg in 5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68135-020-01 1 in 1 CARTON 06/09/2005 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125117 06/09/2005 Labeler - BioMarin Pharmaceutical Inc. (079722386)
Trademark Results [NAGLAZYME]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NAGLAZYME 78819328 3193328 Live/Registered |
BioMarin Pharmaceutical Inc. 2006-02-21 |
 NAGLAZYME 78629370 3132316 Live/Registered |
BioMarin Pharmaceutical Inc. 2005-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.