Meclizine Hydrochloride by Mylan Pharmaceuticals Inc.
Meclizine Hydrochloride by
Drug Labeling and Warnings
Meclizine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MECLIZINE HYDROCHLORIDE- meclizine tablet
Mylan Pharmaceuticals Inc.
----------
DESCRIPTION
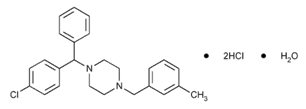
Chemically, meclizine hydrochloride, USP is 1-(p-Chloro-α-phenylbenzyl)-4-(m-methylbenzyl) piperazine dihydrochloride monohydrate. The molecular weight is 481.88 g/mole.
Meclizine hydrochloride tablets, USP are available in two different strengths, 12.5 mg and 25 mg.
Inert ingredients for the tablets are: anhydrous lactose, colloidal silicon dioxide, crospovidone, magnesium stearate and microcrystalline cellulose.
CLINICAL PHARMACOLOGY
Meclizine hydrochloride is an antihistamine that shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
Pharmacokinetics
The available pharmacokinetic information for meclizine following oral administration has been summarized from published literature.
Absorption
Meclizine is absorbed after oral administration with maximum plasma concentrations reaching at a median Tmax value of 3 hours post-dose (range: 1.5 to 6 hours) for the tablet dosage form.
Metabolism
The metabolic fate of meclizine in humans is unknown. In an in vitro metabolic study using human hepatic microsome and recombinant CYP enzyme, CYP2D6 was found to be the dominant enzyme for metabolism of meclizine.
The genetic polymorphism of CYP2D6 that results in extensive-, poor-, intermediate- and ultrarapid metabolizer phenotypes could contribute to large inter-individual variability in meclizine exposure.
INDICATIONS AND USAGE
Meclizine hydrochloride tablets are indicated for the treatment of vertigo associated with diseases affecting the vestibular system.
CONTRAINDICATIONS
Meclizine hydrochloride tablets are contraindicated in individuals who have shown a previous hypersensitivity to it.
WARNINGS
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking this drug.
Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland.
PRECAUTIONS
Pediatric Use
Clinical studies establishing safety and effectiveness in children have not been done; therefore, usage is not recommended in children under 12 years of age.
Pregnancy Use
Pregnancy Category B
Reproduction studies in rats have shown cleft palates at 25-50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine increases the risk of abnormalities when administered during pregnancy. Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine, or any other medication, should be used during pregnancy only if clearly necessary.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when meclizine is administered to a nursing woman.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of meclizine has not been evaluated. As meclizine undergoes metabolism, hepatic impairment may result in increased systemic exposure of the drug. Treatment with meclizine should be administered with caution in patients with hepatic impairment.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of meclizine has not been evaluated. Due to a potential for drug/metabolite accumulation, meclizine should be administered with caution in patients with renal impairment and in the elderly as renal function generally declines with age.
Drug Interactions
There may be increased CNS depression when meclizine is administered concurrently with other CNS depressants, including alcohol, tranquilizers, and sedatives (see WARNINGS).
Based on in vitro evaluation, meclizine is metabolized by CYP2D6. Therefore there is a possibility for a drug interaction between meclizine and CYP2D6 inhibitors.
ADVERSE REACTIONS
Anaphylactoid reaction, drowsiness, dry mouth, headache, fatigue, vomiting and, on rare occasions, blurred vision have been reported.
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
For the treatment of vertigo associated with diseases affecting the vestibular system, the recommended dose is 25 to 100 mg daily, in divided dosage, depending upon clinical response.
HOW SUPPLIED
Meclizine Hydrochloride Tablets, USP are available containing 12.5 mg or 25 mg of meclizine hydrochloride, USP.
The 12.5 mg tablets are white to off-white, round, unscored tablets debossed with M on one side of the tablet and MCZ over 12 on the other side. They are available as follows:
NDC: 0378-5485-77
bottles of 90 tablets
NDC: 0378-5485-10
bottles of 1000 tablets
The 25 mg tablets are white to off-white, round, unscored tablets debossed with M on one side of the tablet and MCZ over 25 on the other side. They are available as follows:
NDC: 0378-5486-77
bottles of 90 tablets
NDC: 0378-5486-10
bottles of 1000 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Revised: 4/2018
MECL:R4
PRINCIPAL DISPLAY PANEL - 12.5 mg
NDC: 0378-5485-77
Meclizine
Hydrochloride
Tablets, USP
12.5 mg
Rx only 90 Tablets
Each tablet contains:
Meclizine
hydrochloride, USP 12.5 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F ).
[See USP Controlled Room
Temperature.]
Usual Dosage: See accompanying
prescribing information.
MOTION SICKNESS: 25 mg to 50 mg
daily.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM5485MM1
PRINCIPAL DISPLAY PANEL - 25 mg
NDC: 0378-5486-77
Meclizine
Hydrochloride
Tablets, USP
25 mg
Rx only 90 Tablets
Each tablet contains:
Meclizine
hydrochloride, USP 25 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F ).
[See USP Controlled Room
Temperature.]
Usual Dosage: See accompanying
prescribing information.
MOTION SICKNESS: 25 mg to 50 mg
daily.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM5486MM1
| MECLIZINE HYDROCHLORIDE
meclizine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MECLIZINE HYDROCHLORIDE
meclizine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Pharmaceuticals Inc. (059295980) |