ATNAA ATROPINE AND PRALIDOXIME CHLORIDE AUTO-INJECTOR- atropine and pralidoxime chloride kit
ATNAA atropine and pralidoxime chloride by
Drug Labeling and Warnings
ATNAA atropine and pralidoxime chloride by is a Prescription medication manufactured, distributed, or labeled by Meridian Medical Technologies, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
The Antidote Treatment - Nerve Agent, Auto-Injector (ATNAA) provides Atropine Injection and Pralidoxime Chloride Injection in separate chambers as sterile, pyrogen-free solutions for intramuscular injection.
The ATNAA is a specially designed unit for automatic self- or buddy-administration by military personnel. When activated, the ATNAA sequentially administers atropine and pralidoxime chloride through a single needle. The recommended procedure (see DOSAGE AND ADMINISTRATION) is to inject the contents of the auto-injector into the muscles of an outer thigh or into the buttocks.
When activated, each ATNAA dispenses:
2.1 mg atropine in 0.7 mL of a sterile, pyrogen-free solution containing 12.47 mg glycerin and not more than 2.8 mg phenol, citrate buffer, and Water for Injection. The pH range is 4.0 – 5.0.
And
600 mg of pralidoxime chloride in 2 mL of a sterile, pyrogen-free solution containing 40 mg benzyl alcohol, 22.5 mg glycine, and Water for Injection. The pH is adjusted with hydrochloric acid. The pH range is 2.0 – 3.0.
After an ATNAA has been activated, the empty container should be disposed of properly (see
DOSAGE AND ADMINISTRATION). It cannot be refilled, nor can the protruding needle be retracted.
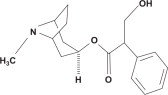
Atropine, an anticholinergic agent (muscarinic antagonist), occurs as white crystals, usually needle- like, or as a white, crystalline powder. It is slightly soluble in water, soluble in glycerin and ether, and freely soluble in alcohol and chloroform with a molecular weight of 289.38. Atropine, a naturally occurring belladonna alkaloid, is a racemic mixture of equal parts of d- and l- hyoscyamine, whose activity is due almost entirely to the levo isomer of the drug. Chemically, atropine is designated as 1αH,5αH-Tropan-3α-ol (±)-tropate. Its empirical formula is C17H23NO3 and its structural formula is:
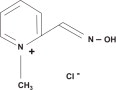
Pralidoxime chloride, a cholinesterase reactivator, is an odorless, white to pale-yellow crystalline powder, freely soluble in water, with a molecular weight of 172.61. Chemically, pralidoxime chloride is designated as 2-formyl-1-methylpyridinium chloride oxime. Its empirical formula is C7H9CIN2O and its structural formula is:
The specific activity of the drug resides in the 2-formyl-1-methylpyridinium ion and is independent of the particular salt employed. The chloride salt is preferred because of physiologic compatibility, excellent water solubility at all temperatures, and high potency per gram, due to its low molecular weight.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
Atropine
Atropine is commonly classified as an anticholinergic or antiparasympathetic (parasympatholytic) drug. More precisely, however, it is termed an antimuscarinic agent since it antagonizes the muscarine-like actions of acetylcholine and other choline esters.
Atropine inhibits the muscarinic actions of acetylcholine on structures innervated by postganglionic cholinergic nerves, and on smooth muscles which respond to endogenous acetylcholine but are not so innervated. As with other antimuscarinic agents, the major action of atropine is a competitive or surmountable antagonism which can be overcome by increasing the concentration of acetylcholine at receptor sites of the effector organ (e.g., by using anticholinesterase agents which inhibit the enzymatic destruction of acetylcholine). The receptors antagonized by atropine are the peripheral structures that are stimulated or inhibited by muscarine, (i.e., exocrine glands and smooth and cardiac muscle).
Responses to postganglionic cholinergic nerve stimulation may also be inhibited by atropine but this occurs less readily than with responses to injected (exogenous) choline esters.
Pralidoxime Chloride
The principal action of pralidoxime is to reactivate cholinesterase (mainly outside the central nervous system) which has been inactivated by phosphorylation due to an organophosphorous nerve agent or related compound, although pralidoxime does not reactivate cholinesterase inactivated by all organophosphate nerve agents (e.g. soman). The destruction of accumulated acetylcholine can then proceed and neuromuscular junctions will again function normally. Pralidoxime also slows the process of “aging” of phosphorylated cholinesterase to a non-reactivatable form and detoxifies certain organophosphates by direct chemical reaction.
Pharmacodynamics:
Atropine
Atropine reduces secretions in the mouth and respiratory passages, relieves the constriction and spasm of the respiratory passages, and may reduce the paralysis of respiration which results from actions of the toxic agent on the central nervous system. Atropine-induced parasympathetic inhibition may be preceded by a transient phase of stimulation, especially on the heart where small doses first slow the rate before characteristic tachycardia develops due to paralysis of vagal control. Although mild vagal excitation occurs, the increased respiratory rate and occasionally increased depth of respiration produced by atropine are more probably the result of bronchiolar dilatation. Accordingly, atropine is an unreliable respiratory stimulant and large or repeated doses may depress respiration.
Adequate doses of atropine abolish various types of reflex vagal cardiac slowing or asystole. The drug also prevents or abolishes bradycardia or asystole produced by injection of choline esters, anticholinesterase agents or other parasympathomimetic drugs, and cardiac arrest produced by stimulation of the vagus. Atropine may also lessen the degree of partial heart block when vagal activity is an etiologic factor. In some patients with complete heart block, the idioventricular rate may be accelerated by atropine; in others, the rate is stabilized. Occasionally, a large dose may cause atrioventricular (A-V) block and nodal rhythm.
Atropine in clinical doses counteracts the peripheral dilatation and abrupt decrease in blood pressure produced by choline esters. However, when given by itself, atropine does not exert a striking or uniform effect on blood vessels or blood pressure. Systemic doses slightly raise systolic and lower diastolic pressures and can produce significant postural hypotension. Such doses also slightly increase cardiac output and decrease central venous pressure. Occasionally, therapeutic doses dilate cutaneous blood vessels, particularly in the “blush” area (atropine flush), and may cause atropine “fever” due to suppression of sweat gland activity in infants and small children.
Pralidoxime Chloride
Pralidoxime chloride has its most critical effect in relieving paralysis of the muscles of respiration. Because pralidoxime is less effective in relieving depression of the respiratory center, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site.
Pralidoxime relieves muscarinic signs and symptoms, salivation, bronchospasm, etc., but this action is relatively unimportant since atropine is adequate for this purpose.
Published reports have established the safety and efficacy of atropine and pralidoxime chloride used separately, as well as the safety and increased efficacy of atropine and pralidoxime chloride when administered concomitantly in the treatment of nerve agent poisoning in humans3.
Pharmacokinetics:
Atropine
Atropine is rapidly and well absorbed after intramuscular administration. Atropine disappears rapidly from the blood and is distributed throughout the various body tissues and fluids. Much of the drug is destroyed by enzymatic hydrolysis, particularly in the liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in various secretions, including milk. Atropine readily crosses the placental barrier and enters the fetal circulation.
The Cmax, Tmax, and T½ of atropine following 2.09 mg atropine given intramuscularly by multi-chambered delivery system was 13 ± 3 ng/mL, 31 ± 30 minutes, and 2.4 ± 0.3 hours, respectively. The protein binding of atropine is 14 to 22% in plasma. There are gender differences in the pharmacokinetics of atropine. The AUC(0-inf) and Cmax were 15% higher in females than males. The half-life of atropine is slightly shorter (approximately 20 minutes) in females than males.
Pralidoxime Chloride
Pralidoxime is distributed throughout the extracellular water; it is not bound to plasma protein. The drug is rapidly excreted in the urine partly unchanged, and partly as a metabolite produced by the liver. Consequently, pralidoxime is relatively short acting and repeated doses may be needed, especially where there is any evidence of continuing absorption of the poison.
The Cmax, Tmax, and T½ of pralidoxime following 600 mg pralidoxime given intramuscularly by multi-chambered delivery system was 7 ± 3 mcg/mL, 28 ± 15 minutes, and 2 ± 1 hour, respectively. The Cmax of pralidoxime was about 36% higher in females than males but the AUC was comparable between the two genders.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
In the face of life-threatening poisoning by organophosphorous nerve agents, there are no absolute contraindications for the use of the ATNAA (see WARNINGS).
-
WARNINGS
While ATNAA can be administered to all individuals with a life-threatening exposure to organophosphorous nerve agents, it should be administered with extreme caution to individuals with the following disorders when the symptoms of nerve agent poisoning are less severe: individuals who are hypersensitive to any component of the product, disorders of heart rhythm such as atrial flutter, severe narrow angle glaucoma, pyloric stenosis, or prostatic hypertrophy.
More than one dose of ATNAA, to a maximum of three doses, may be necessary, especially when exposure is massive or symptoms are severe (see DOSAGE AND ADMINISTRATION). Children are more susceptible than adults to the toxic effects of anticholinergic agents.
Severe difficulty in breathing requires artificial respiration in addition to the use of the ATNAA.
Pralidoxime is not effective in the treatment of poisoning due to phosphorus, inorganic phosphates, or organophosphates not having anticholinesterase activity.
-
PRECAUTIONS
General: The desperate condition of the organophosphorous-poisoned patient will generally mask such minor signs and symptoms of atropine and pralidoxime treatment as have been noted in normal subjects.
Because pralidoxime is excreted in the urine, a decrease in renal function will result in increased blood levels of the drug.
The ATNAA should be used with caution in all individuals over 40 years of age. Conventional systemic doses may precipitate acute glaucoma in susceptible patients, convert partial organic pyloric stenosis into complete pyloric obstruction, precipitate urinary retention in patients with prostatic hypertrophy, or cause inspiration of bronchial secretions and formation of dangerous viscid plugs in patients with chronic lung disease.
Information for Patients: Appropriate steps must be taken to insure that personnel understand the indications for, and use of, the ATNAA, including review of symptoms of poisoning and operation of the ATNAA (see DOSAGE AND ADMINISTRATION and Patient Instruction Sheet).
Drug Interactions: When atropine and pralidoxime are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone because pralidoxime may potentiate the effect of atropine.2,3,4
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of atropine and pralidoxime. Since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions. Morphine, theophylline, aminophylline, succinylcholine, reserpine, and phenothiazine- type tranquilizers should be avoided in treating personnel with organophosphorous poisoning.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No reports regarding the potential of atropine or pralidoxime chloride for carcinogenesis, mutagenesis, or impairment of fertility have been published in the literature. Since the ATNAA is indicated for short-term emergency use only, no investigations of these aspects have been conducted.
Pregnancy: Teratogenic Effects - Pregnancy Category C: Adequate animal reproduction studies have not been conducted with atropine, pralidoxime, or the combination. It is not known whether pralidoxime or atropine can cause fetal harm when administered to a pregnant woman or if these agents can affect reproductive capacity. The ATNAA should be administered to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Mild to moderate pain may be experienced at the site of injection.
Atropine
The major side effects of atropine can be attributed to antimuscarinic action. These include dryness of the mouth, blurred vision, photophobia, confusion, headache, dizziness, tachycardia, palpitations, flushing, urinary hesitance or retention, constipation, abdominal distention, nausea, vomiting, loss of libido, and impotency. Anhidrosis may produce heat intolerance and impairment of temperature regulation in a hot environment. Larger or toxic doses may produce such central effects as restlessness, tremor, fatigue, locomotor difficulties, delirium, followed by hallucinations, depression,
and ultimately, medullary paralysis and death. Large doses can also lead to circulatory collapse. In such cases, blood pressure declines and death due to respiratory failure may ensue following paralysis and coma. Hypersensitivity reactions will occasionally occur with atropine; these are usually seen as skin rashes, on occasion progressing to exfoliation.
Pralidoxime Chloride
Pralidoxime may cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure, hyperventilation, decreased renal function, and muscular weakness when given parenterally to normal volunteers who have not been exposed to anticholinesterase poisons. In actual cases of poisoning, it is very difficult to differentiate some of the toxic effects produced by atropine or the organophosphate compound from those of pralidoxime chloride.
Excitement and manic behavior immediately following recovery of consciousness after organophosphorous poisoning treated with pralidoxime chloride have been reported in several cases. However, similar behavior has occurred in cases that were not treated with pralidoxime. 3,5,6
Elevations in SGOT and/or SGPT enzyme levels were observed in one of six normal volunteers given 1200 mg of pralidoxime chloride intramuscularly, and in 4 of 6 volunteers given 1800 mg intramuscularly. Levels returned to normal in about two weeks.
Transient elevations in creatine phosphokinase were observed in all normal volunteers given the drug. A single intramuscular injection of 330 mg in 1 mL in rabbits caused myonecrosis, inflammation, and hemorrhage.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
Symptoms:
Atropine
Serious overdosage with atropine is characterized by widespread paralysis of parasympathetically innervated organs. Dry mucous membranes, widely dilated and nonresponsive pupils, tachycardia, fever, and cutaneous flush are especially prominent, as are mental and neurological symptoms.
Disorientation, mania, hallucinations, gait disturbances, and symptoms may last 48 hours or longer. In instances of severe intoxication, respiratory depression, coma, circulatory collapse, and death may occur.
The fatal dose of atropine is not known. In the treatment of organophosphorous poisoning, 200 mg doses have been used and doses as high as 1000 mg have been given.
In children, 10 mg or less may be fatal. With a dose as low as 0.5 mg, undesirable minimal symptoms or responses of overdosage may occur. These increase in severity and extent with larger doses of the drug (excitement, hallucinations, delirium, and coma with a dose of 10 mg or more). However, in the presence of organophosphate poisoning, higher doses of atropine may be tolerated.
Pralidoxime Chloride
Symptoms of pralidoxime chloride overdose have been observed in normal subjects only: dizziness, blurred vision, diplopia, headache, impaired accommodation, nausea, slight tachycardia. In therapy it has been difficult to differentiate side effects due to the drug from those due to effects of the poison.
Treatment:
Supportive treatment should be administered as indicated. If respiration is depressed, artificial respiration with oxygen is necessary. Ice bags, alcohol sponges or a hypothermia blanket may be required to reduce fever, especially in children. Catheterization may be necessary if urinary retention occurs. Since atropine elimination takes place through the kidney, urinary output must be maintained and increased if possible; intravenous fluids may be indicated. Because of the affected person's photophobia, the room should be darkened.
In the event of toxic overdosage, a short acting barbiturate or diazepam may be given as needed to control marked excitement and convulsions. Large doses for sedation should be avoided because central depressant action may coincide with the depression occurring late in atropine poisoning.
Central stimulants are not recommended. Physostigmine, given as an atropine antidote by slow intravenous injection of 1 to 4 mg (0.5 to 1.0 mg in children), rapidly abolishes delirium and coma caused by large doses of atropine. Since physostigmine has a short duration of action, the patient may again lapse into coma after one or two hours and repeated doses are likely to be required.
Neostigmine, pilocarpine, and methacholine are of little real benefit, since they do not penetrate the blood-brain barrier.
-
DOSAGE AND ADMINISTRATION
For optimal reactivation of organophosphorous-inhibited cholinesterase, the ATNAA should be administered as soon as possible after appearance of symptoms of nerve agent poisoning (see below).
The ATNAA should be self- or buddy–administered by military personnel after donning protective mask and hood at the first sign of a chemical attack, and only if some or all of the following mild symptoms of nerve agent exposure are present:
- - Unexplained runny nose
- - Unexplained sudden headache
- - Sudden drooling
- - Difficulty in seeing (dimness of vision and miosis)
- - Tightness of chest or difficulty in breathing
- - Wheezing and coughing
- - Localized sweating and muscular twitching in the area of contaminated skin
- - Stomach cramps
- - Nausea, with or without vomiting
- - Tachycardia followed by bradycardia
The following are the instructions that should be given to military personnel.
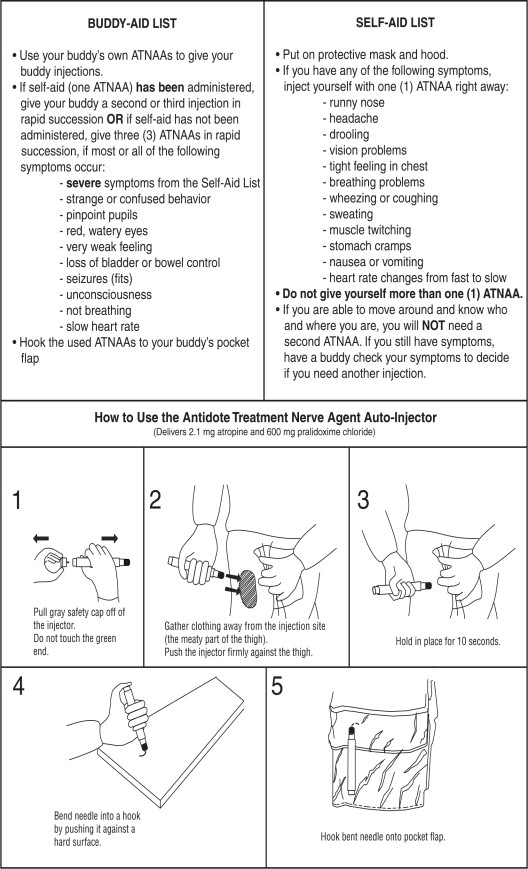
Self-Aid
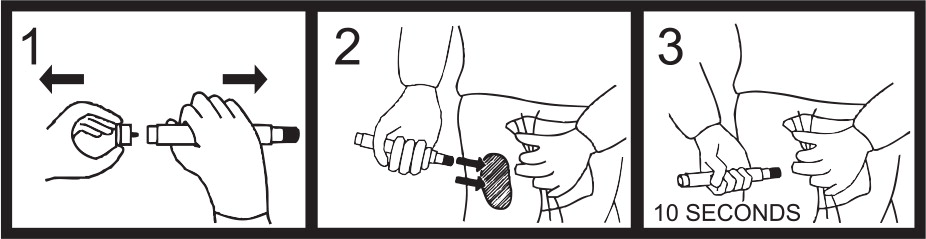
- Administer one (1) ATNAA into your lateral thigh muscle or buttocks as follows:
- Remove gray safety cap from back end.
- Place front end on outer thigh and push hard until injector functions. Hold firmly in place for ten seconds.
- Using a hard surface, bend needle into hook. Push ejected needle through a pocket flap (or other thick and conspicuous part of outer clothing).
- Wait 10 to 15 minutes for the antidote to take effect. If you are able to ambulate, know who you are, and where you are, you will NOT need a second injection. Warning: Giving yourself a second set of injections may cause an overdose of the ATNAA which could result in incapacitation.
- If symptoms of nerve agent poisoning are not relieved after administering one injection, seek someone else to check your symptoms. A buddy must administer the second and third injections, if needed.
Buddy-Aid
- Casualties with severe symptoms may experience most or all of the mild symptoms described above, plus most or all of the following:
- - Strange or confused behavior
- - Increased wheezing and increased difficulty in breathing
- - Severely pinpointed pupils
- - Red eyes with tearing
- - Vomiting
- - Severe muscular twitching and general weakness
- - Involuntary urination and defecation
- - Convulsions
- - Unconsciousness
- - Respiratory failure
- - Bradycardia
- If you encounter a service member suffering from severe signs of nerve agent poisoning, render the following aid:
- Mask the casualty, if necessary. Do not fasten the hood.
- If self-aid (one ATNAA) has been administered, administer in rapid succession two (2) additional ATNAAs into the casualty's lateral thigh muscle or buttocks.
Note: Use the casualty's own ATNAAs when providing aid. Do not use your own injectors on a casualty. If you do, you may not have any antidote available when needed for self- aid. - If self-aid (one ATNAA) has not been administered, administer in rapid succession three (3) ATNAAs into the casualty's lateral thigh muscle or buttocks.
IMPORTANT: PHYSICIANS AND/OR MEDICAL PERSONNEL ASSISTING EVACUATED VICTIMS OF NERVE AGENTS, SHOULD AVOID EXPOSING THEMSELVES TO CONTAMINATION BY THE VICTIM'S CLOTHING.
-
HOW SUPPLIED
The Antidote Treatment – Nerve Agent, Auto-Injector (ATNAA) provides Atropine Injection (atropine, 2.1 mg/0.7 mL) and Pralidoxime Chloride Injection (pralidoxime chloride, 600 mg/2 mL) in sterile solutions for intramuscular injection. The ATNAA is a self-contained unit designed for automatic self- or buddy-administration by military personnel. ATNAAs are supplied through the Directorate of Medical Materiel, Defense Supply Center, Philadelphia.
Store at 25°C (77°F); excursions permitted to 15 - 30°C (59 - 86°F)
[see USP Controlled Room Temperature]
Keep from Freezing. Protect from Light.
Manufactured by:
MERIDIAN MEDICAL TECHNOLOGIES®, INC.
A wholly-owned subsidiary of King Pharmaceuticals®, Inc.0001566
4/10Rx only.
-
REFERENCES
- Landauer, W: Cholinomimetic teratogens. V. The effect of oximes and related cholinesterase reactivators. Teratology 15: 33 (Feb) 1977.
- Moller, K.O., Jensen-Holm, J. and Lausen, H.H.: Ugeskr Laeg. 123: 501, 1961.
- Namba, T., Nolte, C.T., Jackrel, J. and Grob, D.: Poisoning due to organophosphate insecticides. Acute and chronic manifestations. Amer. J. Med. 50: 475 (Apr), 1971.
- Arena, J.M.: Poisoning, Toxicology Symptoms, Treatments, ed. 4, Springfield, IL, Charles C. Thomas, 1979, p. 133.
- Brachfeld, J., and Zavon, M.R.: Organic phosphate (Phosdrin®) Intoxication. Report of a case and the results of treatment with 2-PAM, Arch. Environ. Health 11:859, 1965.
- Hayes, W.J., Jr.: Toxicology of Pesticides. Baltimore, The Williams & Wilkins Company, 1975, p. 416.
------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------
- PATIENT PACKAGE INSERT
-
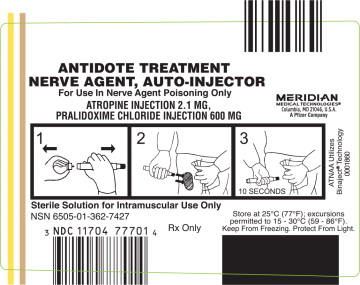
PRINCIPAL DISPLAY PANEL
Principal Display Panel
ANTIDOTE TREATMENT NERVE AGENT, AUTO-INJECTOR
For Use in Nerve Agent Poisoning Only
ATROPINE INJECTION 2.1 MG,
PRALIDOXIME CHLORIDE INJECTION 600MG
MERIDIAN MEDICAL TECHNOLOGIES®
Columbia, MD 21046, USA
A Pfizer Company.
ATNAA Utilizes Binaject® Technology 0001860
Sterile Solution for Intramuscular Use Only
NSN 6505-01-362-7427
NDC: 11704-777-01
Rx Only
Store at 25°C (77°F); excursions permitted to 15 - 30°C (59-86°F).
Keep From Freezing. Protect From Light.
-
INGREDIENTS AND APPEARANCE
ATNAA ATROPINE AND PRALIDOXIME CHLORIDE AUTO-INJECTOR
atropine and pralidoxime chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11704-777 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11704-777-01 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 01/17/2002 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 0.7 mL Part 2 1 SYRINGE, GLASS 2 mL Part 1 of 2 ATNAA ATROPINE AND PRALIDOXIME CHLORIDE AUTO-INJECTOR
atropine injectionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength atropine (UNII: 7C0697DR9I) (atropine - UNII:7C0697DR9I) atropine 2.1 mg in 0.7 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Citric Acid Monohydrate (UNII: 2968PHW8QP) Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Sodium Citrate (UNII: 1Q73Q2JULR) Nitrogen (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.7 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021175 01/17/2002 Part 2 of 2 ATNAA ATROPINE AND PRALIDOXIME CHLORIDE AUTO-INJECTOR
pralidoxime chloride injectionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pralidoxime chloride (UNII: 38X7XS076H) (pralidoxime - UNII:P7MU9UTP52) pralidoxime chloride 600 mg in 2 mL Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021175 01/17/2002 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021175 01/17/2002 Labeler - Meridian Medical Technologies, Inc. (167671341) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 038889234 MANUFACTURE(11704-777) , ANALYSIS(11704-777) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 078808315 MANUFACTURE(11704-777) , LABEL(11704-777) , PACK(11704-777) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 167671341 MANUFACTURE(11704-777) , LABEL(11704-777) , PACK(11704-777) , ANALYSIS(11704-777)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.