POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE- dextrose, sodium chloride, and potassium chloride injection
Potassium Chloride in Dextrose and Sodium Chloride by
Drug Labeling and Warnings
Potassium Chloride in Dextrose and Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
(See chart below for quantitative information.)
Potassium Chloride in Dextrose and Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents. These products are intended for intravenous administration.
The formulas of the active ingredients are:
Ingredients Molecular
FormulaMolecular
WeightSodium Chloride USP
Potassium Chloride USPNaCl
KCl58.44
74.55Hydrous Dextrose USP 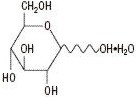
198.17 Composition – Each 100 mL contains: Solution Hydrous Dextrose USP Sodium Chloride USP Potassium Chloride USP Concentration of Electrolytes (mEq/liter) Calories per
literCalculated
Osmolarity
mOsmol/literpH Sodium Potassium Chloride Water for Injection USP qs 0.15% Potassium Chloride in 5% Dextrose and
0.20% Sodium Chloride Injection USP5 g 0.2 g 0.15 g 34 20 54 170 360 4.4 (3.5–6.5) 0.15% Potassium Chloride in 5% Dextrose and
0.33% Sodium Chloride Injection USP5 g 0.33 g 0.15 g 56 20 76 170 405 4.4 (3.5–6.5) 0.075% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP5 g 0.45 g 0.075 g 77 10 87 170 425 4.4 (3.5–6.5) 0.15% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP5 g 0.45 g 0.15 g 77 20 97 170 445 4.4 (3.5–6.5) 0.22% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP5 g 0.45 g 0.22 g 77 30 107 170 465 4.4 (3.5–6.5) 0.30% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP5 g 0.45 g 0.3 g 77 40 117 170 490 4.4 (3.5–6.5) 0.15% Potassium Chloride in 5% Dextrose and
0.9% Sodium Chloride Injection USP5 g 0.9 g 0.15 g 154 20 174 170 600 4.4 (3.5–6.5) Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
Addition of medication should be accomplished using complete aseptic technique.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector and the other is a medication addition site. Refer to the Directions for Use of the container.
-
CLINICAL PHARMACOLOGY
These intravenous solutions provide electrolytes and calories, and are a source of water for hydration. They are capable of inducing diuresis depending on the clinical condition of the patient.
Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance, and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid.
Potassium, the principal cation of intracellular fluid, participates in carbohydrate utilization and protein synthesis, and is critical in the regulation of nerve conduction and muscle contraction, particularly in the heart.
Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
Dextrose provides a source of calories. Dextrose is readily metabolized, may decrease losses of body protein and nitrogen, promotes glycogen deposition and decreases or prevents ketosis if sufficient doses are provided.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there is sodium retention with edema.
In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
Solutions containing potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure, and in conditions in which potassium retention is present.
-
PRECAUTIONS
General
These solutions should be used with care in patients with hypervolemia, renal insufficiency, urinary tract obstruction, or impending or frank cardiac decompensation.
Extraordinary electrolyte losses such as may occur during protracted nasogastric suction, vomiting, diarrhea or gastrointestinal fistula drainage may necessitate additional electrolyte supplementation.
Additional essential electrolytes, minerals and vitamins should be supplied as needed.
Sodium-containing solutions should be administered with caution to patients receiving corticosteroids or corticotropin, or to other salt-retaining patients.
Care should be exercised in administering solutions containing sodium or potassium to patients with renal or cardiovascular insufficiency, with or without congestive heart failure, particularly if they are postoperative or elderly.
Potassium therapy should be guided primarily by serial electrocardiograms, especially in patients receiving digitalis. Serum potassium levels are not necessarily indicative of tissue potassium levels. Solutions containing potassium should be used with caution in the presence of cardiac disease, particularly when accompanied by renal disease.
Solutions containing dextrose should be used with caution in patients with overt or known subclinical diabetes mellitus, or carbohydrate intolerance for any reason.
To minimize the risk of possible incompatibilities arising from mixing any of these solutions with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Do not use plastic containers in series connection.
If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
These solutions are intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours. Use only if solution is clear and container and seals are intact.
Laboratory Tests
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation. Significant deviations from normal concentrations may require tailoring of the electrolyte pattern, in these or alternative solutions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Potassium Chloride in Dextrose and Sodium Chloride Injections USP have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with Potassium Chloride in Dextrose and Sodium Chloride Injections USP. It is also not known whether Potassium Chloride in Dextrose and Sodium Chloride Injections USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium Chloride in Dextrose and Sodium Chloride Injections USP should be given to a pregnant woman only if clearly needed.
Labor and Delivery
The effects of Potassium Chloride in Dextrose and Sodium Chloride Injections USP on the duration of labor or delivery, on the possibility that forceps delivery or other intervention or resuscitation of the newborn will be necessary, and on the later growth, development, and functional maturation of the child are unknown.
As reported in the literature, potassium containing solutions have been administered during labor and delivery. Caution should be exercised, and the fluid balance, glucose and electrolyte concentrations, and acid-base balance, of both mother and fetus should be evaluated periodically or whenever warranted by the condition of the patient or fetus.
Nursing Mothers
It is not known whether these drugs are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Potassium Chloride in Dextrose and Sodium Chloride Injections USP are administered to a nursing woman.
Pediatric Use
Safety and effectiveness of Potassium Chloride in Dextrose and Sodium Chloride Injections USP in pediatric patients have not been established by adequate and well-controlled studies. However, as referenced in the medical literature, potassium chloride injection has been used to treat pediatric patients with potassium deficiency when oral replacement therapy is not feasible.
For patients receiving potassium supplement at greater than maintenance rates, frequent monitoring of serum potassium levels and serial EKGs are recommended.
Dextrose is safe and effective for the stated indications in pediatric patients (see INDICATIONS AND USAGE). As reported in the literature, the dosage selection and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia. Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In neonates or in very small infants even small volumes of fluid may affect fluid and electrolyte balance. Care must be exercised in treatment of neonates, especially pre-term neonates, whose renal function may be immature and whose ability to excrete fluid and solute loads may be limited. Fluid intake, urine output, and serum electrolytes should be monitored closely.
See WARNINGS and DOSAGE AND ADMINISTRATION.
Geriatric Use
Clinical studies of Potassium Chloride in Dextrose and Sodium Chloride Injections USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
These drugs are known to be substantially excreted by the kidney, and the risk of toxic reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
See WARNINGS.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
Too rapid infusion of hypertonic solutions may cause local pain and venous irritation. Rate of administration should be adjusted according to tolerance. Use of the largest peripheral vein and a small bore needle is recommended. (See DOSAGE AND ADMINISTRATION.)
Symptoms may result from an excess or deficit of one or more of the ions present in the solution; therefore, frequent monitoring of electrolyte levels is essential.
Hypernatremia may be associated with edema and exacerbation of congestive heart failure due to the retention of water, resulting in an expanded extracellular fluid volume.
Reactions reported with the use of potassium-containing solutions include nausea, vomiting, abdominal pain and diarrhea. The signs and symptoms of potassium intoxication include paresthesias of the extremities, areflexia, muscular or respiratory paralysis, mental confusion, weakness, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities and cardiac arrest.
Potassium deficits result in disruption of neuromuscular function, and intestinal ileus and dilatation.
If infused in large amounts, chloride ions may cause a loss of bicarbonate ions, resulting in an acidifying effect.
The physician should also be alert to the possibility of adverse reaction to drug additives. Prescribing information for drug additives to be administered in this manner should be consulted.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of fluid overload during parenteral therapy, reevaluate the patient's condition, and institute appropriate corrective treatment.
In the event of overdosage with potassium-containing solutions, discontinue the infusion immediately and institute corrective therapy to reduce serum potassium levels.
Treatment of hyperkalemia includes the following:
- Dextrose Injection USP, 10% or 25%, containing 10 units of crystalline insulin per 20 grams of dextrose administered intravenously, 300 to 500 mL per hour.
- Absorption and exchange of potassium using sodium or ammonium cycle cation exchange resin, orally and as retention enema.
- Hemodialysis and peritoneal dialysis. The use of potassium-containing foods or medications must be eliminated. However, in cases of digitalization, too rapid a lowering of plasma potassium concentration can cause digitalis toxicity.
-
DOSAGE AND ADMINISTRATION
These solutions are for intravenous use only.
Dosage is to be directed by a physician and is dependent upon age, weight, clinical condition of the patient and laboratory determinations. Frequent laboratory determinations and clinical evaluation are essential to monitor changes in blood glucose and electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
When a hypertonic solution is to be administered peripherally, it should be slowly infused through a small bore needle, placed well within the lumen of a large vein to minimize venous irritation. Carefully avoid infiltration.
Usually, up to 40 mEq of potassium per liter daily is sufficient to replace normal loss in adults. Typical infusion rates should not exceed 10 mEq per hour or 120 mEq per day. Pediatric patients may require 2 to 3 mEq per kg of body weight daily. See WARNINGS and PRECAUTIONS for pediatric use.
Fluid administration should be based on calculated maintenance or replacement fluid requirements for each patient.
Dextrose may be administered to normal individuals at a rate of 0.5 g/kg/hour without producing glycosuria. At the maximum infusion rate of 0.8 g/kg/hour, approximately 95% of the dextrose is retained.
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Potassium Chloride in Dextrose and Sodium Chloride Injections USP are supplied in EXCEL® Containers. The 1000 mL containers are packaged 12 per case and the 250 mL containers are packaged 24 per case.
Canada DIN NDC REF Size 0.15% Potassium Chloride in 5% Dextrose and
0.20% Sodium Chloride Injection USP
(20 mEq K+/liter)01931598 0264-7645-00 L6450 1000 mL 0264-7645-20 L6452 250 mL 0.15% Potassium Chloride in 5% Dextrose and
0.33% Sodium Chloride Injection USP
(20 mEq K+/liter)01931601 0264-7655-00 L6550 1000 mL 0.075% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP
(10 mEq K+/liter)0264-7634-00 L6340 1000 mL 0.15% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP
(20 mEq K+/liter)01931547 0264-7635-00 L6350 1000 mL 0.22% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP
(30 mEq K+/liter)0264-7636-00 L6360 1000 mL 0.30% Potassium Chloride in 5% Dextrose and
0.45% Sodium Chloride Injection USP
(40 mEq K+/liter)01931571 0264-7638-00 L6380 1000 mL 0.15% Potassium Chloride in 5% Dextrose and
0.9% Sodium Chloride Injection USP
(20 mEq K+/liter)01931644 0264-7652-00 L6520 1000 mL Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C).
Storage in automated dispensing machines: Brief exposure up to 2 weeks to ultraviolet or fluorescent light does not adversely affect the product labeling legibility; prolonged exposure can cause fading of the red label. Rotate stock frequently.
- SPL UNCLASSIFIED SECTION
-
Directions for Use of EXCEL® Container
Caution: Do not use plastic containers in series connection.
To Open
Tear overwrap down at notch and remove solution container.
Check for minute leaks by squeezing solution container firmly.
If leaks are found, discard solution as sterility may be impaired.
If supplemental medication is desired, follow directions below before preparing for administration.NOTE: Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter. Any container which is suspect should not be used.
- Use only if solution is clear and container and seals are intact.
Preparation for Administration
- Remove plastic protector from sterile set port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
To Add Medication
Warning: Some additives may be incompatible.
To Add Medication Before Solution Administration
- Prepare medication site.
- Using syringe with 18–22 gauge needle, puncture medication port and inner diaphragm and inject.
- Squeeze and tap ports while ports are upright and mix solution and medication thoroughly.
To Add Medication During Solution Administration
- Close clamp on the set.
- Prepare medication site.
- Using syringe with 18–22 gauge needle of appropriate length (at least 5/8 inch), puncture resealable medication port and inner diaphragm and inject.
- Remove container from IV pole and/or turn to an upright position.
- Evacuate both ports by tapping and squeezing them while container is in the upright position.
- Mix solution and medication thoroughly.
- Return container to in use position and continue administration.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.075% Potassium Chloride in
5% Dextrose and
0.45% Sodium Chloride
Injection USPREF L6340
NDC: 0264-7634-00
1000 mL
EXCEL® CONTAINER10 mEq K+/liter
Y94-003-346 LD-258-3Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.45 g; Potassium Chloride USP 0.075 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 425 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 77; K+ 10; Cl– 87
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Y94-003-285
LD-166-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.15% Potassium Chloride in
5% Dextrose and
0.45% Sodium Chloride
Injection USPREF L6350
NDC: 0264-7635-00
DIN 019315471000 mL
EXCEL® CONTAINER20 mEq K+/liter
Y94-003-347 LD-265-4Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.45 g; Potassium Chloride USP 0.15 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 445 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 77; K+ 20; Cl– 97
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-299
LD-158-4EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.22% Potassium Chloride in
5% Dextrose and
0.45% Sodium Chloride
Injection USPREF L6360
NDC: 0264-7636-001000 mL
EXCEL® CONTAINER30 mEq K+/liter
Y94-003-348 LD-266-3Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.45 g; Potassium Chloride USP 0.22 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 465 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 77; K+ 30; Cl– 107
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862Y94-003-283
LD-157-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.30% Potassium Chloride in
5% Dextrose and
0.45% Sodium Chloride
Injection USPREF L6380
NDC: 0264-7638-00
DIN 019315711000 mL
EXCEL® CONTAINER40 mEq K+/liter
Y94-003-349 LD-274-3Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.45 g; Potassium Chloride USP 0.3 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 490 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 77; K+ 40; Cl– 117
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-281
LD-156-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.15% Potassium Chloride in
5% Dextrose and
0.20% Sodium Chloride
Injection USPREF L6450
NDC: 0264-7645-00
DIN 019315981000 mL
EXCEL® CONTAINER20 mEq K+/liter
Y94-003-350 LD-506-2Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.2 g; Potassium Chloride USP 0.15 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 360 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 34; K+ 20; Cl– 54
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.
Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-279
LD-163-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 250 mL Container Label
0.15% Potassium Chloride in
5% Dextrose and 0.20% Sodium
Chloride Injection USPREF L6452
NDC: 0264-7645-20
DIN 01931598250 mL
EXCEL® CONTAINER20 mEq K+/liter
Y94-003-274 LD-267-2Each 100 mL contains: Hydrous Dextrose USP 5 g;
Sodium Chloride USP 0.2 g; Potassium Chloride USP 0.15 g;
Water for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 360 mOsmol/liter, hypertonic
Electrolytes (mEq/liter): Na+ 34; K+ 20; Cl– 54
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear and
container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid excessive
heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-273
LD-162-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.15% Potassium Chloride in
5% Dextrose and
0.9% Sodium Chloride
Injection USPREF L6520
NDC: 0264-7652-00
DIN 019316441000 mL
EXCEL® CONTAINER20 mEq K+/liter
Y94-003-351 LD-504-2Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.9 g; Potassium Chloride USP 0.15 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 600 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 154; K+ 20; Cl– 174
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-275
LD-155-3EXP LOT

-
PRINCIPAL DISPLAY PANEL - 1000 mL Container Label
0.15% Potassium Chloride in
5% Dextrose and
0.33% Sodium Chloride
Injection USPREF L6550
NDC: 0264-7655-00
DIN 019316011000 mL
EXCEL® CONTAINER20 mEq K+/liter
Y94-003-352 LD-505-2Each 100 mL contains: Hydrous Dextrose USP 5 g; Sodium
Chloride USP 0.33 g; Potassium Chloride USP 0.15 g; Water
for Injection USP qspH: 4.4 (3.5-6.5); Calc. Osmolarity: 405 mOsmol/liter,
hypertonicElectrolytes (mEq/liter): Na+ 56; K+ 20; Cl– 76
Sterile, nonpyrogenic. Single dose container. Do not use in series
connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.WARNINGS: Some additives may be incompatible. Consult with
pharmacist. When introducing additives, use aseptic techniques.
Mix thoroughly. Do not store.Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap,
check for minute leaks by squeezing container firmly. If leaks are found,
discard solution as sterility may be impaired.Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4Y94-003-277
LD-159-3EXP LOT

-
INGREDIENTS AND APPEARANCE
POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7645 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.2 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.15 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7645-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC: 0264-7645-10 24 in 1 CASE 02/17/1988 08/06/2004 2 500 mL in 1 CONTAINER; Type 0: Not a Combination Product 3 NDC: 0264-7645-20 24 in 1 CASE 02/17/1988 3 250 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7655 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.33 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.15 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7655-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7634 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.45 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.075 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7634-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7635 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.45 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.15 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7635-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7636 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.45 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.22 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7636-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7638 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.45 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7638-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 POTASSIUM CHLORIDE IN DEXTROSE AND SODIUM CHLORIDE
dextrose, sodium chloride, and potassium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-7652 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE (UNII: IY9XDZ35W2) (DEXTROSE - UNII:IY9XDZ35W2) DEXTROSE 5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 0.9 g in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 0.15 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-7652-00 12 in 1 CASE 02/17/1988 1 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019630 02/17/1988 Labeler - B. Braun Medical Inc. (002397347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.