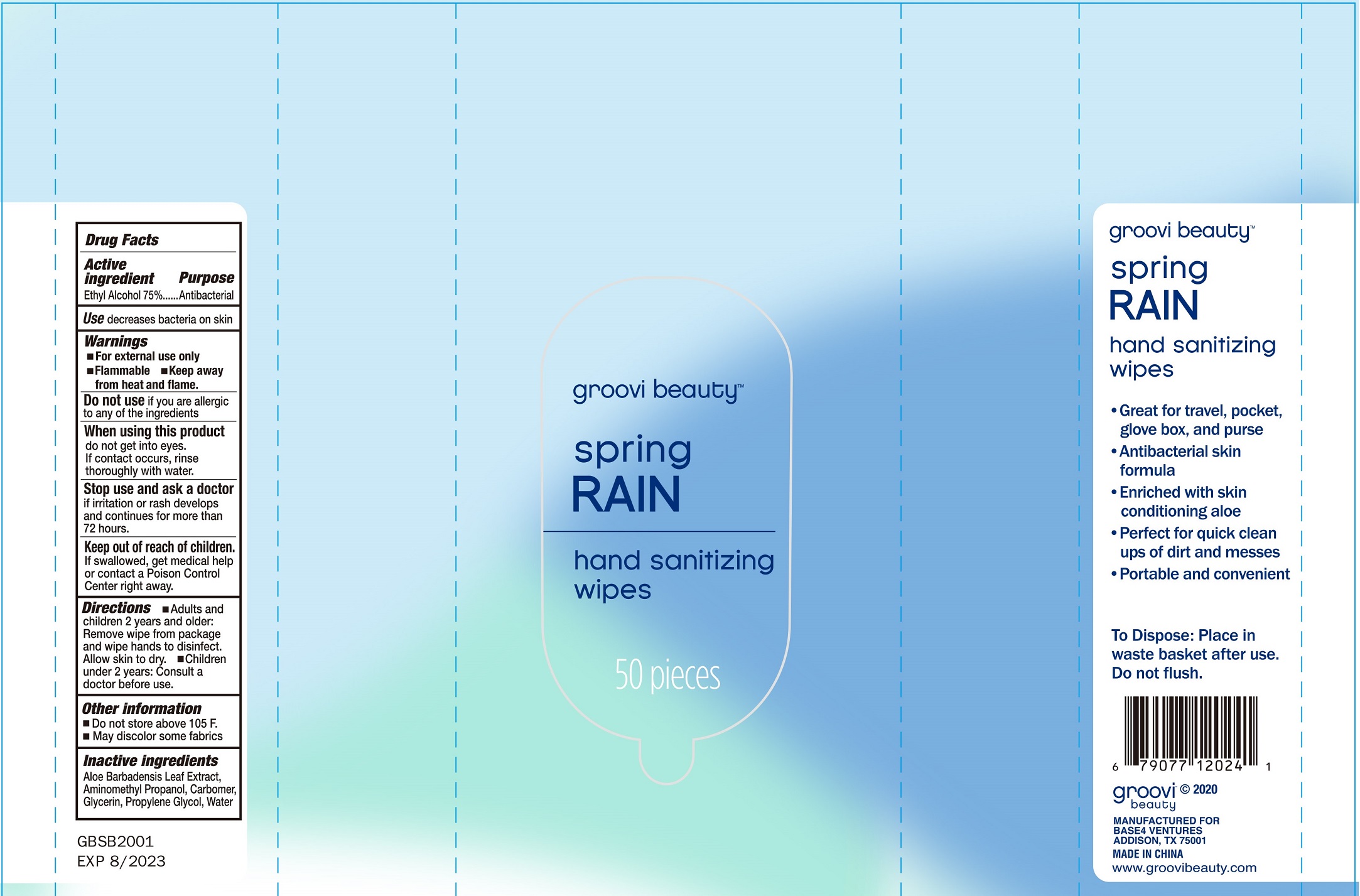
groovi beauty spring RAIN hand sanitizing wips by HAINING LILY CLEANING PRODUCTS CO., LTD Antibacterial Wipes
groovi beauty spring RAIN hand sanitizing wips by
Drug Labeling and Warnings
groovi beauty spring RAIN hand sanitizing wips by is a Otc medication manufactured, distributed, or labeled by HAINING LILY CLEANING PRODUCTS CO., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GROOVI BEAUTY SPRING RAIN HAND SANITIZING WIPS- alcohol swab
HAINING LILY CLEANING PRODUCTS CO., LTD
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Antibacterial Wipes
Warning
- For external use only.
- Flammable, keep away from heat and flame
- Do not sue if you are allergic to any of the ingredients.
- When uising this product do not get into eyes, if contact occurs, rinse thoroughly with water.
- Stop use and ask a doctor if irritation or rash develops and continues for more than 72 hours.
Do not store above 105℉.
May discolor some fabrics.
direction
- Adults and children 2 years and older: Remove wipe fro package and wipe hands to disinfect. Allow skin to dry.
- Children under 2 years: consult a doctor before use.
| GROOVI BEAUTY SPRING RAIN HAND SANITIZING WIPS
alcohol swab |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HAINING LILY CLEANING PRODUCTS CO., LTD (654463293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HAINING LILY CLEANING PRODUCTS CO., LTD | 654463293 | manufacture(77435-110) | |
Revised: 7/2020
Document Id: e14d4a63-d907-41f7-8aba-82f6ee7c771d
Set id: 5a282421-b016-45a8-b0ab-f13721acabf6
Version: 10
Effective Time: 20200728
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.