STAY AWAKE- caffeine tablet
Stay Awake by
Drug Labeling and Warnings
Stay Awake by is a Otc medication manufactured, distributed, or labeled by CHAIN DRUG MARKETING ASSOCIATION INC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heartbeat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
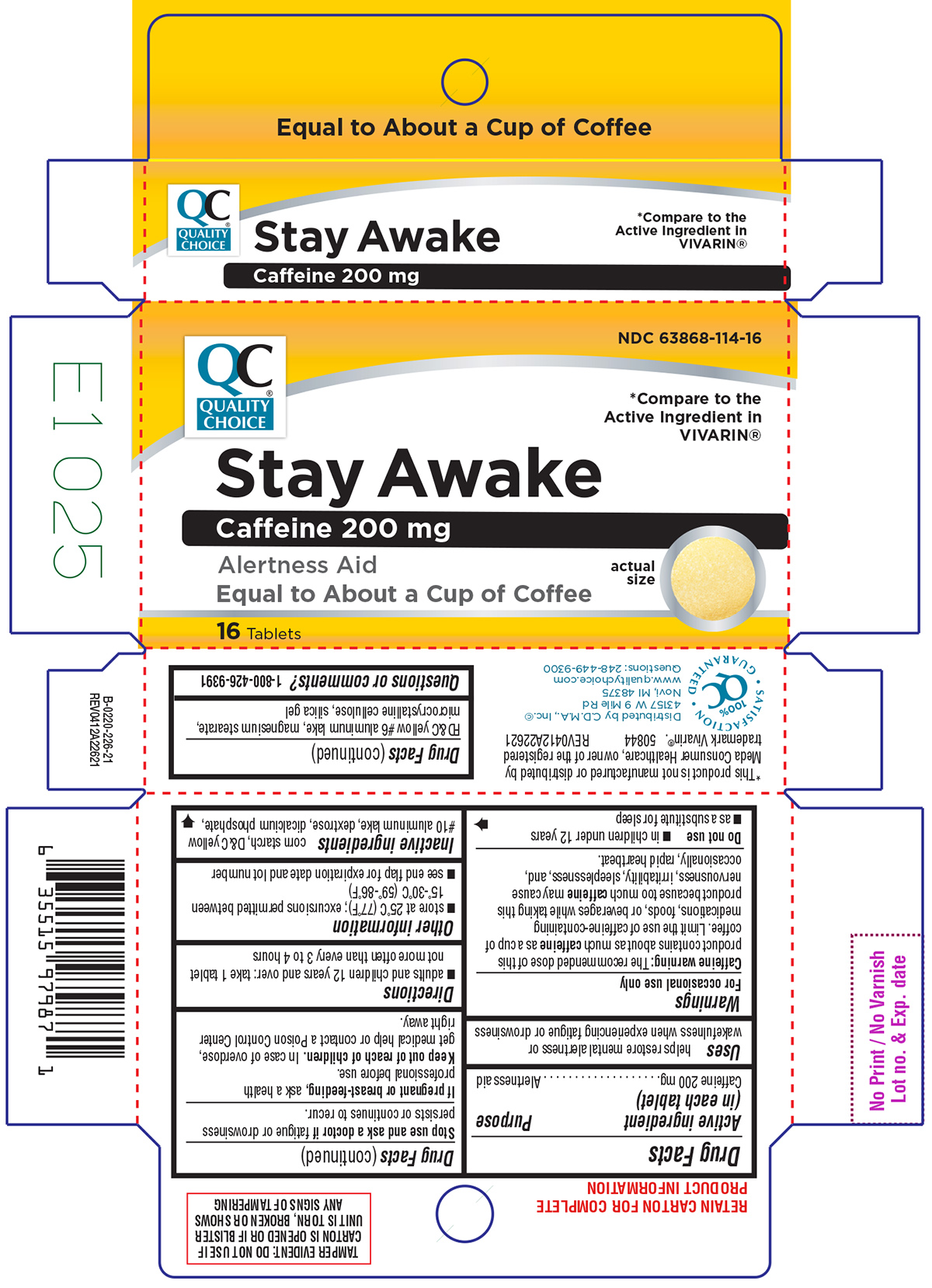
Principal Display Panel
NDC: 63868-114-16
QC®
QUALITY
CHOICE*Compare to the
Active ingredient in
VIVARIN®Stay Awake
Caffeine 200 mg
Alertness Aid
Equal to About a Cup of Coffeeactual
size16 Tablets
*This product is not manufactured or distributed by Meda Consumer Healthcare, owner of the registered trademark Vivarin®.
50844 REV0412A22621SATISFACTION
GUARANTEED
100%
QCDistributed by C.D.M.A., Inc©
43157 W 9 Mile Rd
Novi, MI 48375
www.qualitychoice.com
Questions: 248-449-9300TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Quality Choice 44-226
-
INGREDIENTS AND APPEARANCE
STAY AWAKE
caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 63868-114 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DEXTROSE (UNII: IY9XDZ35W2) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color YELLOW Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;226 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63868-114-40 5 in 1 CARTON 11/21/1996 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 63868-114-16 2 in 1 CARTON 11/21/1996 2 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part340 11/21/1996 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC (011920774) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(63868-114) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(63868-114) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(63868-114) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(63868-114) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(63868-114)
Trademark Results [Stay Awake]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STAY AWAKE 85047007 not registered Dead/Abandoned |
A.N.N.A. LLC 2010-05-25 |
 STAY AWAKE 76575252 not registered Dead/Abandoned |
Pharmaceutical Formulations, Inc. 2004-02-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.