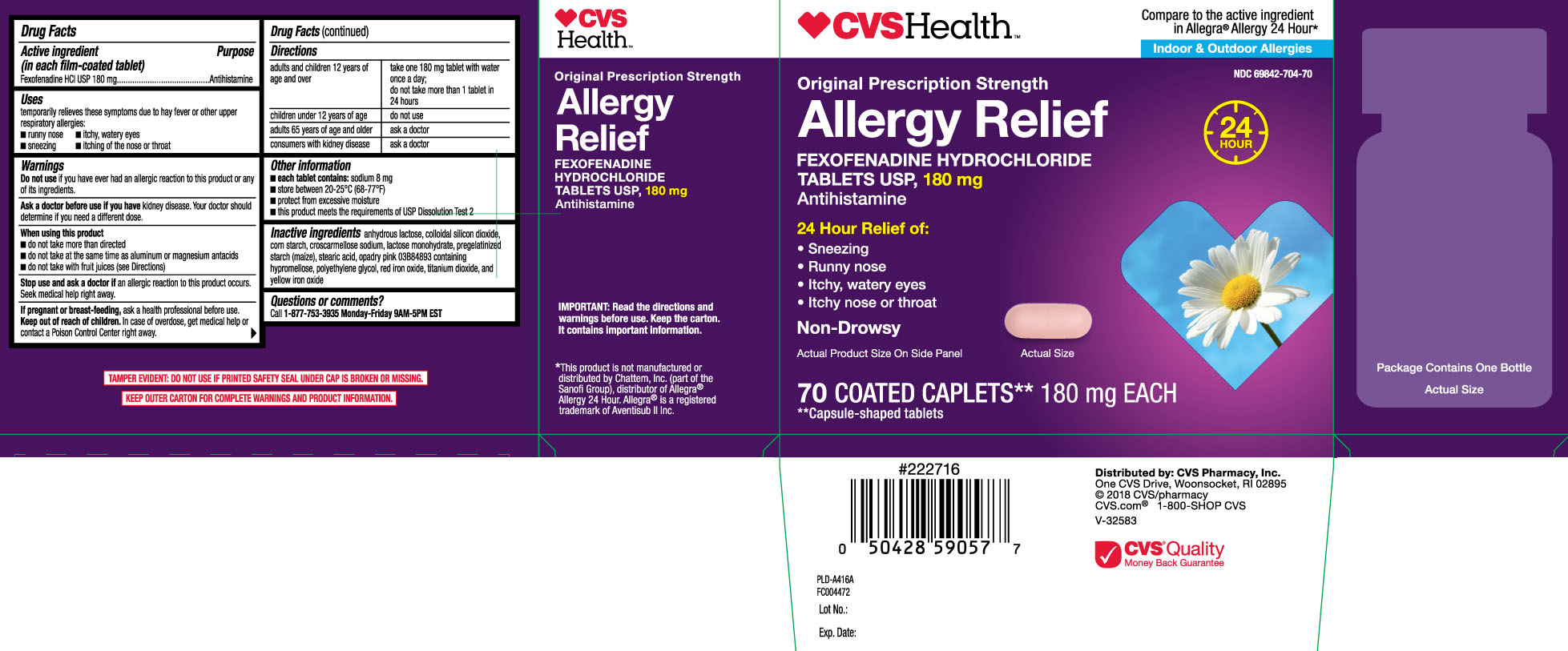
ALLERGY RELIEF- fexofenadine hcl tablet, coated
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each film-coated tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to the active ingredient in Allegra® Allergy 24 hour*
Original Prescription Strength
Allergy Relief
FEXOFENADINE HYDROCHLORIDE
TABLETS USP, 180 mg
Antihistamine
24 Hour Relief of:
- sneezing
- runny nose
- itchy, watery eyes
- itchy nose or throat
Non-Drowsy
COATED CAPLETS**
**Capsule-shaped tablets
IMPORTANT: Read the directions and warnings before use. Keep the carton. It contains inportant information.
*This product is not manufactured or distributed by Chattem Inc., (part of the Sanofi group).distributor of Allegra® Allergy 24 hour. Allegra® is a registered trademark of AventisubII Inc.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket,RI 02895
CVS.com®
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
fexofenadine hcl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69842-704 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 17mm Flavor Imprint Code SG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69842-704-70 1 in 1 BOX 10/31/2017 1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079112 10/31/2017 Labeler - CVS Pharmacy (062312574)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.