PROMESCENT- lidicaine spray
Promescent by
Drug Labeling and Warnings
Promescent by is a Otc medication manufactured, distributed, or labeled by Absorption, Swiss-American CDMO, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
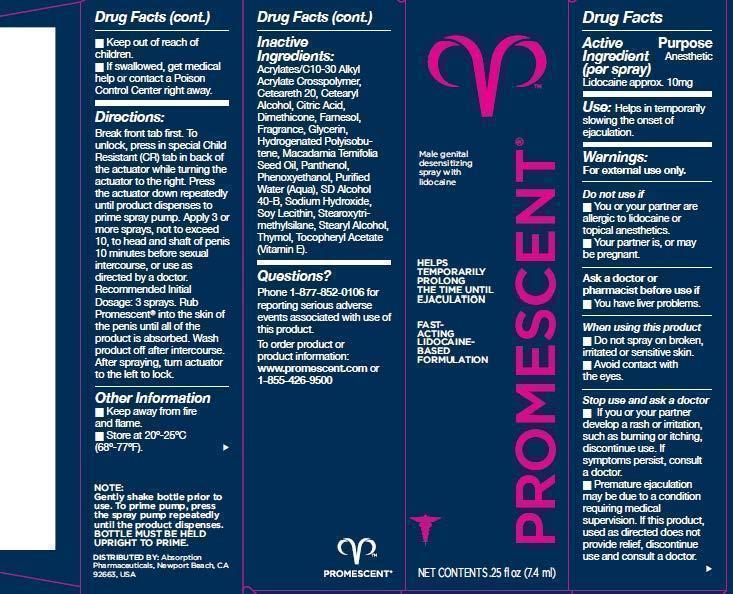
- Active Ingredient (per spray)
- Purpose
- Keep out of reach of children
- Use:
-
Warnings:
For external use only.
Do not use if
- You or your partner are allergic to lidocaine or topical anesthetics.
- Your partner is, or may be pregnant.
Ask a doctor or pharmacist before use if
- You have liver problems.
When using this product
- Do not spray on broken, irritated or sensitive skin.
- Avoid contact with the eyes.
Stop use and ask a doctor
- If you or your partner develop a rash or irritation, such as burning or itching, dicontinue use. If symptoms persist, consult a doctor.
- Premature ejaculation may be due to a condition requiring medical supervision. If this product, used as directed does not provide relief, discontinue use and consult a doctor.
-
Directions:
Break front tab first. To unlock, press in special Child Resistant (R) tab in back of the actuator while turning the actuator to the right. Press the actuator down repeatedly until product dispenses to prime spray pump. Apply 3 or more sprays, not to exceed 10, to head and shaft of penis 10 minutes before sexual intercourse, or use as directed by a doctor. Recommended Initial Dosage: 3 sprays. Rub Promescent into the skin of the penis until all of the product is absorbed. Wash product off after intersourse. After spraying, turn actuator to the left to lock.
-
Inactive Ingredients:
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Ceteareth-20, Cetearyl Alcohol, Citric Acid, Dimethicone, Farnesol, Fragrance, Glycerin, Hydrogenated Polyisobutene, Macadamia Ternifolia Seed Oil, Panthenol, Phenoxyethanol, Purified Water (Aqua), SD Alcohol 40-B, Sodium Hydroxide, Soy Lecithin, Stearoxytrimethylsilane, Stearyl Alcohol, Thymol, Tocopheryl Acetate (Vitamin E).
- Labeling
-
INGREDIENTS AND APPEARANCE
PROMESCENT
lidicaine sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55636-590 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) Lidocaine 10 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) GLYCERIN (UNII: PDC6A3C0OX) PANTHENOL (UNII: WV9CM0O67Z) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) STEAROXYTRIMETHYLSILANE (UNII: 9862TW94B2) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) MACADAMIA OIL (UNII: 515610SU8C) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) FARNESOL (UNII: EB41QIU6JL) THYMOL (UNII: 3J50XA376E) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55636-590-74 1 in 1 CARTON 01/11/2013 1 7.4 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC: 55636-590-13 1 in 1 CARTON 01/11/2013 2 1.3 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 3 NDC: 55636-590-26 1 in 1 CARTON 01/11/2013 3 3.8 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 4 NDC: 55636-590-52 1 in 1 CARTON 01/11/2013 4 5.4 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 01/11/2013 Labeler - Absorption (014937753) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(55636-590) , label(55636-590) , pack(55636-590)
Trademark Results [Promescent]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROMESCENT 88524677 not registered Live/Pending |
Absorption Pharmaceuticals, LLC 2019-07-19 |
 PROMESCENT 88524356 not registered Live/Pending |
Absorption Pharmaceuticals, LLC 2019-07-19 |
 PROMESCENT 77551399 3793979 Live/Registered |
ABSORPTION PHARMACEUTICALS, LLC 2008-08-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.