TIBSOVO- ivosidenib tablet, film coated
Tibsovo by
Drug Labeling and Warnings
Tibsovo by is a Prescription medication manufactured, distributed, or labeled by Agios Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TIBSOVO safely and effectively. See full prescribing information for TIBSOVO.
TIBSOVO® (ivosidenib tablets), for oral use
Initial U.S. Approval: 2018WARNING: DIFFERENTIATION SYNDROME
See full prescribing information for complete boxed warning.
Patients treated with TIBSOVO have experienced symptoms of differentiation syndrome, which can be fatal if not treated. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution (5.1, 6.1).
INDICATIONS AND USAGE
TIBSOVO is an isocitrate dehydrogenase-1 (IDH1) inhibitor indicated for the treatment of acute myeloid leukemia (AML) with a susceptible IDH1 mutation as detected by an FDA-approved test in:
DOSAGE AND ADMINISTRATION
500 mg orally once daily with or without food until disease progression or unacceptable toxicity (2.2). Avoid a high-fat meal.
DOSAGE FORMS AND STRENGTHS
Tablets: 250 mg (3).
CONTRAINDICATIONS
None (4).
WARNINGS AND PRECAUTIONS
- QTc Interval Prolongation: Monitor electrocardiograms and electrolytes. If QTc interval prolongation occurs, dose reduce or withhold, then resume dose or permanently discontinue TIBSOVO (2.3, 5.2).
- Guillain-Barré Syndrome: Monitor patients for signs and symptoms of new motor and/or sensory findings. Permanently discontinue TIBSOVO in patients who are diagnosed with Guillain-Barré syndrome (2.3, 5.3).
ADVERSE REACTIONS
The most common adverse reactions (≥20%) were fatigue, arthralgia, leukocytosis, diarrhea, edema, nausea, dyspnea, mucositis, electrocardiogram QT prolonged, rash, cough, decreased appetite, myalgia, constipation, and pyrexia (6.1).
The most common laboratory abnormalities (≥20%) were hemoglobin decreased, calcium decreased, sodium decreased, magnesium decreased, uric acid increased, potassium decreased, alkaline phosphatase increased, aspartate aminotransferase increased, phosphate decreased, and creatinine increased (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Agios Pharmaceuticals at 1-833-228-8474 and or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong or Moderate CYP3A4 Inhibitors: Reduce TIBSOVO dose with strong CYP3A4 inhibitors. Monitor patients for increased risk of QTc interval prolongation (2.4, 5.2, 7.1, 12.3).
- Strong CYP3A4 Inducers: Avoid concomitant use with TIBSOVO (7.1, 12.3).
- Sensitive CYP3A4 substrates: Avoid concomitant use with TIBSOVO (7.2, 12.3).
- QTc Prolonging Drugs: Avoid concomitant use with TIBSOVO. If co-administration is unavoidable, monitor patients for increased risk of QTc interval prolongation (5.2, 7.1).
USE IN SPECIFIC POPULATIONS
Lactation: Advise women not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DIFFERENTIATION SYNDROME
1 INDICATIONS AND USAGE
1.1 Newly-Diagnosed Acute Myeloid Leukemia
1.2 Relapsed or Refractory Acute Myeloid Leukemia
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Monitoring and Dose Modifications for Toxicities
2.4 Dose Modification for Use with Strong CYP3A4 Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
5.2 QTc Interval Prolongation
5.3 Guillain-Barré Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Ivosidenib
7.2 Effect of Ivosidenib on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Newly-Diagnosed AML
14.2 Relapsed or Refractory AML
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME
Patients treated with TIBSOVO have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, hypoxia, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, hypotension, and hepatic, renal, or multi-organ dysfunction. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
-
1 INDICATIONS AND USAGE
1.1 Newly-Diagnosed Acute Myeloid Leukemia
TIBSOVO is indicated for the treatment of newly-diagnosed acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test in adult patients who are ≥ 75 years old or who have comorbidities that preclude use of intensive induction chemotherapy [see Dosage and Administration (2.1), Clinical Pharmacology (12.1) and Clinical Studies (14.1)].
1.2 Relapsed or Refractory Acute Myeloid Leukemia
TIBSOVO is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation as detected by an FDA-approved test [see Dosage and Administration (2.1), Clinical Pharmacology (12.1) and Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of AML with TIBSOVO based on the presence of IDH1 mutations in the blood or bone marrow [see Clinical Studies (14.1)]. Patients without IDH1 mutations at diagnosis should be retested at relapse because a mutation in IDH1 may emerge during treatment and at relapse. Information on FDA-approved tests for the detection of IDH1 mutations in AML is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dose of TIBSOVO is 500 mg taken orally once daily until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treat for a minimum of 6 months to allow time for clinical response.
Administer TIBSOVO with or without food. Do not administer TIBSOVO with a high-fat meal because of an increase in ivosidenib concentration [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Do not split or crush TIBSOVO tablets. Administer TIBSOVO tablets orally about the same time each day. If a dose of TIBSOVO is vomited, do not administer a replacement dose; wait until the next scheduled dose is due. If a dose of TIBSOVO is missed or not taken at the usual time, administer the dose as soon as possible and at least 12 hours prior to the next scheduled dose. Return to the normal schedule the following day. Do not administer 2 doses within 12 hours.
Patients with the Comorbidities of Severe Renal or Severe Hepatic Impairment
Treatment with TIBSOVO has not been studied in patients with pre-existing severe renal or hepatic impairment. For patients with pre-existing severe renal or hepatic impairment, consider the risks and potential benefits before initiating treatment with TIBSOVO [see Use in Specific Populations (8.6, 8.7)].
2.3 Monitoring and Dose Modifications for Toxicities
Assess blood counts and blood chemistries prior to the initiation of TIBSOVO, at least once weekly for the first month, once every other week for the second month, and once monthly for the duration of therapy. Monitor blood creatine phosphokinase weekly for the first month of therapy. Monitor electrocardiograms (ECGs) at least once weekly for the first 3 weeks of therapy and then at least once monthly for the duration of therapy. Manage any abnormalities promptly [see Adverse Reactions (6.1)].
Interrupt dosing or reduce dose for toxicities. See Table 1 for dose modification guidelines.
Table 1. Recommended Dose Modifications for TIBSOVO *Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, Grade 4 is life-threatening.
Adverse Reactions Recommended Action - Differentiation syndrome
- If differentiation syndrome is suspected, administer systemic corticosteroids and initiate hemodynamic monitoring until symptom resolution and for a minimum of 3 days [see Warnings and Precautions (5.1)].
- Interrupt TIBSOVO if severe signs and/or symptoms persist for more than 48 hours after initiation of systemic corticosteroids [see Warnings and Precautions (5.1)]
- Resume TIBSOVO when signs and symptoms improve to Grade 2* or lower.
- Noninfectious leukocytosis (white blood cell [WBC] count greater than 25 x 109/L or an absolute increase in total WBC of greater than 15 x 109/L from baseline)
- Initiate treatment with hydroxyurea, as per standard institutional practices, and leukapheresis if clinically indicated.
- Taper hydroxyurea only after leukocytosis improves or resolves.
- Interrupt TIBSOVO if leukocytosis is not improved with hydroxyurea, and then resume TIBSOVO at 500 mg daily when leukocytosis has resolved.
- QTc interval greater than 480 msec to 500 msec
- Monitor and supplement electrolyte levels as clinically indicated [see Warnings and Precautions (5.2)].
- Review and adjust concomitant medications with known QTc interval-prolonging effects [see Drug Interactions (7.1)].
- Interrupt TIBSOVO
- Restart TIBSOVO at 500 mg once daily after the QTc interval returns to less than or equal to 480 msec.
- Monitor ECGs at least weekly for 2 weeks following resolution of QTc prolongation.
- QTc interval greater than 500 msec
- Monitor and supplement electrolyte levels as clinically indicated [see Warnings and Precautions (5.2)].
- Review and adjust concomitant medications with known QTc interval-prolonging effects [see Drug Interactions (7.1)]
- Interrupt TIBSOVO
- Resume TIBSOVO at a reduced dose of 250 mg once daily when QTc interval returns to within 30 msec of baseline or less than or equal to 480 msec.
- Monitor ECGs at least weekly for 2 weeks following resolution of QTc prolongation.
- Consider re-escalating the dose of TIBSOVO to 500 mg daily if an alternative etiology for QTc prolongation can be identified.
- QTc interval prolongation with signs/symptoms of life-threatening arrhythmia
- Discontinue TIBSOVO permanently [see Warnings and Precautions (5.2)].
- Guillain-Barré syndrome
- Discontinue TIBSOVO permanently [see Warnings and Precautions (5.3)].
- Other Grade 3* or higher toxicity considered related to treatment
- Interrupt TIBSOVO until toxicity resolves to Grade 2* or lower.
- Resume TIBSOVO at 250 mg once daily; may increase to 500 mg once daily if toxicities resolve to Grade 1* or lower.
- If Grade 3* or higher toxicity recurs, discontinue TIBSOVO.
2.4 Dose Modification for Use with Strong CYP3A4 Inhibitors
If a strong CYP3A4 inhibitor must be coadministered, reduce the TIBSOVO dose to 250 mg once daily. If the strong inhibitor is discontinued, increase the TIBSOVO dose (after at least 5 half-lives of the strong CYP3A4 inhibitor) to the recommended dose of 500 mg once daily.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Differentiation Syndrome
In the clinical trial, 25% (7/28) of patients with newly diagnosed AML and 19% (34/179) of patients with relapsed or refractory AML treated with TIBSOVO experienced differentiation syndrome. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells and may be life-threatening or fatal if not treated. Symptoms of differentiation syndrome in patients treated with TIBSOVO included noninfectious leukocytosis, peripheral edema, pyrexia, dyspnea, pleural effusion, hypotension, hypoxia, pulmonary edema, pneumonitis, pericardial effusion, rash, fluid overload, tumor lysis syndrome and creatinine increased. Of the 7 patients with newly diagnosed AML who experienced differentiation syndrome, 6 (86%) patients recovered. Of the 34 patients with relapsed or refractory AML who experienced differentiation syndrome, 27 (79%) patients recovered after treatment or after dose interruption of TIBSOVO. Differentiation syndrome occurred as early as 1 day and up to 3 months after TIBSOVO initiation and has been observed with or without concomitant leukocytosis.
If differentiation syndrome is suspected, initiate dexamethasone 10 mg IV every 12 hours (or an equivalent dose of an alternative oral or IV corticosteroid) and hemodynamic monitoring until improvement [see Dosage and Administration (2.3)]. If concomitant noninfectious leukocytosis is observed, initiate treatment with hydroxyurea or leukapheresis, as clinically indicated. Taper corticosteroids and hydroxyurea after resolution of symptoms and administer corticosteroids for a minimum of 3 days. Symptoms of differentiation syndrome may recur with premature discontinuation of corticosteroid and/or hydroxyurea treatment. If severe signs and/or symptoms persist for more than 48 hours after initiation of corticosteroids, interrupt TIBSOVO until signs and symptoms are no longer severe [see Dosage and Administration (2.3)].
5.2 QTc Interval Prolongation
Patients treated with TIBSOVO can develop QT (QTc) prolongation [see Clinical Pharmacology (12.2)] and ventricular arrhythmias. Of the 258 patients with hematological malignancies treated with TIBSOVO in the clinical trial, 9% were found to have a QTc interval greater than 500 msec and 14% of patients had an increase from baseline QTc greater than 60 msec. One patient developed ventricular fibrillation attributed to TIBSOVO. The clinical trial excluded patients with baseline QTc of ≥ 450 msec (unless the QTc ≥ 450 msec was due to a pre-existing bundle branch block) or with a history of long QT syndrome or uncontrolled or significant cardiovascular disease.
Concomitant use of TIBSOVO with drugs known to prolong the QTc interval (e.g., anti-arrhythmic medicines, fluoroquinolones, triazole anti-fungals, 5-HT3 receptor antagonists) and CYP3A4 inhibitors may increase the risk of QTc interval prolongation [see Drug Interactions (7.1), Clinical Pharmacology (12.2)]. Conduct monitoring of electrocardiograms (ECGs) and electrolytes [see Dosage and Administration (2.3)].
In patients with congenital long QTc syndrome, congestive heart failure, electrolyte abnormalities, or those who are taking medications known to prolong the QTc interval, more frequent monitoring may be necessary.
Interrupt TIBSOVO if QTc increases to greater than 480 msec and less than 500 msec. Interrupt and reduce TIBSOVO if QTc increases to greater than 500 msec. Permanently discontinue TIBSOVO in patients who develop QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see Dosage and Administration (2.3)].
5.3 Guillain-Barré Syndrome
Guillain-Barré syndrome occurred in < 1% (2/258) of patients treated with TIBSOVO in the clinical study. Monitor patients taking TIBSOVO for onset of new signs or symptoms of motor and/or sensory neuropathy such as unilateral or bilateral weakness, sensory alterations, paresthesias, or difficulty breathing. Permanently discontinue TIBSOVO in patients who are diagnosed with Guillain-Barré syndrome [see Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Differentiation Syndrome [see Warnings and Precautions (5.1)]
- QTc Interval Prolongation [see Warnings and Precautions (5.2)]
- Guillain-Barré Syndrome [see Warnings and Precautions (5.3)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TIBSOVO as a single agent at 500 mg daily was evaluated in 213 patients with AML in Study AG120-C-001 [see Clinical Studies (14.1 and 14.2)]. The median age of TIBSOVO treated patients was 68 (range 18-87) with 68% ≥ 65 years, 51% male, 66% White, 6% Black or African American, 3% Asian, 0.5% Native Hawaiian or other Pacific Islander, 0.5% American Indian or Alaska Native, and 24% other/not provided. Among the 213 patients who received TIBSOVO, 37% were exposed for 6 months or longer and 14% were exposed for 12 months or longer. The most common adverse reactions including laboratory abnormalities in ≥ 20% of 213 patients who received TIBSOVO were hemoglobin decreased, fatigue, arthralgia, calcium decreased, sodium decreased, leukocytosis, diarrhea, magnesium decreased, edema, nausea, dyspnea, uric acid increased, potassium decreased, alkaline phosphatase increased, mucositis, aspartate aminotransferase increased, phosphatase decreased, electrocardiogram QT prolonged, rash, creatinine increased, cough, decreased appetite, myalgia, constipation, and pyrexia.
Newly-Diagnosed AML
The safety profile of single-agent TIBSOVO was studied in 28 adults with newly-diagnosed AML treated with 500 mg daily [see Clinical Studies (14.1)]. The median duration of exposure to TIBSOVO was 4.3 months (range 0.3 to 40.9 months). Ten patients (36%) were exposed to TIBSOVO for at least 6 months and 6 patients (21%) were exposed for at least 1 year.
Common (≥ 5%) serious adverse reactions included differentiation syndrome (18%), electrocardiogram QT prolonged (7%), and fatigue (7%). There was one case of posterior reversible encephalopathy syndrome (PRES).
Common (≥ 10%) adverse reactions leading to dose interruption included electrocardiogram QT prolonged (14%) and differentiation syndrome (11%). Two (7%) patients required a dose reduction due to electrocardiogram QT prolonged. One patient each required permanent discontinuation due to diarrhea and PRES.
The most common adverse reactions reported in the trial are shown in Table 2.
Table 2: Adverse Reactions Reported in ≥ 10% (Any Grade) or ≥ 5% (Grade ≥ 3) of Patients with Newly-Diagnosed AML 1 Grouped term includes leukocytosis, hyperleukocytosis, and increased white blood cell count.
2 Differentiation syndrome can be associated with other commonly reported events such as peripheral edema, leukocytosis, pyrexia, dyspnea, pleural effusion, hypotension, hypoxia, pulmonary edema, pneumonia, pericardial effusion, rash, fluid overload, tumor lysis syndrome, and creatinine increased.
3 Grouped term includes abdominal pain, upper abdominal pain, abdominal discomfort, and abdominal tenderness.
4 Grouped term includes aphthous ulcer, esophageal pain, esophagitis, gingival pain, gingivitis, mouth ulceration, mucosal inflammation, oral pain, oropharyngeal pain, proctalgia, and stomatitis.
5 Grouped term includes asthenia and fatigue.
6 Grouped term includes edema, face edema, fluid overload, fluid retention, hypervolemia, peripheral edema, and swelling face.
7 Grouped term includes arthralgia, back pain, musculoskeletal stiffness, neck pain, and pain in extremity.
8 Grouped term includes myalgia, muscular weakness, musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, and myalgia intercostal.
9 Grouped term includes burning sensation, lumbosacral plexopathy, neuropathy peripheral, paresthesia, and peripheral motor neuropathy.
10 Grouped term includes dyspnea, dyspnea exertional, hypoxia, and respiratory failure.
11 Grouped term includes cough, productive cough, and upper airway cough syndrome.
12 Grouped term includes dermatitis acneiform, dermatitis, rash, rash maculo-papular, urticaria, rash erythematous, rash macular, rash pruritic, rash generalized, rash papular, skin exfoliation, and skin ulcer.
TIBSOVO
(500 mg daily)
N=28Body System
Adverse ReactionAll Grades
n (%)Grade ≥ 3
n (%)Blood System and Lymphatic System Disorders Leukocytosis1 10 (36) 2 (7) Differentiation Syndrome2 7 (25) 3 (11) Gastrointestinal Disorders Diarrhea 17 (61) 2 (7) Nausea 10 (36) 2 (7) Abdominal pain3 8 (29) 1 (4) Constipation 6 (21) 1 (4) Vomiting 6 (21) 1 (4) Mucositis4 6 (21) 0 Dyspepsia 3 (11) 0 General Disorders and Administration Site Conditions Fatigue5 14 (50) 4 (14) Edema6 12 (43) 0 Investigations Electrocardiogram QT prolonged 6 (21) 3 (11) Weight decreased 3 (11) 0 Metabolism and Nutrition Disorders Decreased appetite 11 (39) 1 (4) Musculoskeletal and Connective Tissue Disorders Arthralgia7 9 (32) 1 (4) Myalgia8 7 (25) 1 (4) Nervous System Disorders Dizziness 6 (21) 0 Neuropathy9 4 (14) 0 Headache 3 (11) 0 Respiratory, Thoracic and Mediastinal Disorders Dyspnea10 8 (29) 1 (4) Cough11 4 (14) 0 Skin and Subcutaneous Tissue Disorders Pruritis 4 (14) 1 (4) Rash12 4 (14) 1 (4) Changes in selected post-baseline laboratory values that were observed in patients with newly diagnosed AML are shown in Table 3.
Table 3: Most Common (≥ 10%) or ≥ 5% (Grade ≥ 3) New or Worsening Laboratory Abnormalities Reported in Patients with Newly-Diagnosed AML1 1 Laboratory abnormality is defined as new or worsened by at least one grade from baseline, or if baseline is unknown.
TIBSOVO (500 mg daily)
N=28Parameter All Grades
n (%)Grade ≥ 3
n (%)Hemoglobin decreased 15 (54) 12 (43) Alkaline phosphatase increased 13 (46) 0 Potassium decreased 12 (43) 3 (11) Sodium decreased 11 (39) 1 (4) Uric acid increased 8 (29) 1 (4) Aspartate aminotransferase increased 8 (29) 1 (4) Creatinine increased 8 (29) 0 Magnesium decreased 7 (25) 0 Calcium decreased 7 (25) 1 (4) Phosphate decreased 6 (21) 2 (7) Alanine aminotransferase increased 4 (14) 1 (4) Relapsed or Refractory AML
The safety profile of single-agent TIBSOVO was studied in 179 adults with relapsed or refractory AML treated with 500 mg daily [see Clinical Studies (14.2)].
The median duration of exposure to TIBSOVO was 3.9 months (range 0.1 to 39.5 months). Sixty-five patients (36%) were exposed to TIBSOVO for at least 6 months and 16 patients (9%) were exposed for at least 1 year.
Serious adverse reactions (≥ 5%) were differentiation syndrome (10%), leukocytosis (10%), and electrocardiogram QT prolonged (7%). There was one case of progressive multifocal leukoencephalopathy (PML).
The most common adverse reactions leading to dose interruption were electrocardiogram QT prolonged (7%), differentiation syndrome (3%), leukocytosis (3%) and dyspnea (3%). Five out of 179 patients (3%) required a dose reduction due to an adverse reaction. Adverse reactions leading to a dose reduction included electrocardiogram QT prolonged (1%), diarrhea (1%), nausea (1%), decreased hemoglobin (1%), and increased transaminases (1%). Adverse reactions leading to permanent discontinuation included Guillain-Barré syndrome (1%), rash (1%), stomatitis (1%), and creatinine increased (1%).
The most common adverse reactions reported in the trial are shown in Table 4.
Table 4: Adverse Reactions Reported in ≥ 10% (Any Grade) or ≥ 5% (Grade ≥ 3) of Patients with Relapsed or Refractory AML 1 Grouped term includes leukocytosis, hyperleukocytosis, and increased white blood cell count.
2 Differentiation syndrome can be associated with other commonly reported events such as peripheral edema, leukocytosis, pyrexia, dyspnea, pleural effusion, hypotension, hypoxia, pulmonary edema, pneumonia, pericardial effusion, rash, fluid overload, tumor lysis syndrome, and creatinine increased.
3 Grouped term includes aphthous ulcer, esophageal pain, esophagitis, gingival pain, gingivitis, mouth ulceration, mucosal inflammation, oral pain, oropharyngeal pain, proctalgia, and stomatitis.
4 Grouped term includes vomiting and retching.
5 Grouped term includes abdominal pain, upper abdominal pain, abdominal discomfort, and abdominal tenderness.
6 Grouped term includes asthenia and fatigue.
7 Grouped term includes peripheral edema, edema, fluid overload, fluid retention, and face edema.
8 Grouped term includes angina pectoris, chest pain, chest discomfort, and non-cardiac chest pain.
9 Grouped term includes arthralgia, back pain, musculoskeletal stiffness, neck pain, and pain in extremity.
10 Grouped term includes myalgia, muscular weakness, musculoskeletal pain, musculoskeletal chest pain, musculoskeletal discomfort, and myalgia intercostal.
11 Grouped term includes ataxia, burning sensation, gait disturbance, Guillain-Barré syndrome, neuropathy peripheral, paresthesia, peripheral sensory neuropathy, peripheral motor neuropathy, and sensory disturbance.
12 Grouped term includes cough, productive cough, and upper airway cough syndrome.
13 Grouped term includes dyspnea, respiratory failure, hypoxia, and dyspnea exertional.
14 Grouped term includes dermatitis acneiform, dermatitis, rash, rash maculo-papular, urticaria, rash erythematous, rash macular, rash pruritic, rash generalized, rash papular, skin exfoliation, and skin ulcer.
15 Grouped term includes hypotension and orthostatic hypotension.
TIBSOVO (500 mg daily)
N=179Body System All Grades Grade ≥ 3 Adverse Reaction n (%) n (%) Blood System and Lymphatic System Disorders Leukocytosis1 68 (38) 15 (8) Differentiation Syndrome2 34 (19) 23 (13) Gastrointestinal Disorders Diarrhea 60 (34) 4 (2) Nausea 56 (31) 1 (1) Mucositis3 51 (28) 6 (3) Constipation 35 (20) 1 (1) Vomiting4 32 (18) 2 (1) Abdominal pain5 29 (16) 2 (1) General Disorders and Administration Site Conditions Fatigue6 69 (39) 6 (3) Edema7 57 (32) 2 (1) Pyrexia 41 (23) 2 (1) Chest pain8 29 (16) 5 (3) Investigations Electrocardiogram QT prolonged 46 (26) 18 (10) Metabolism and Nutrition Disorders Decreased appetite 33 (18) 3 (2) Tumor lysis syndrome 14 (8) 11 (6) Musculoskeletal and Connective Tissue Disorders Arthralgia9 64 (36) 8 (4) Myalgia10 33 (18) 1 (1) Nervous System Disorders Headache 28 (16) 0 Neuropathy11 21 (12) 2 (1) Respiratory, Thoracic and Mediastinal Disorders Cough12 40 (22) 1 (<1) Dyspnea13 59 (33) 16 (9) Pleural effusion 23 (13) 5 (3) Skin and Subcutaneous Tissue Disorders Rash14 46 (26) 4 (2) Vascular Disorders Hypotension15 22 (12) 7 (4) Changes in selected post-baseline laboratory values that were observed in patients with relapsed or refractory AML are shown in Table 5.
Table 5: Most Common (≥ 10%) or ≥ 5% (Grade ≥ 3) New or Worsening Laboratory Abnormalities Reported in Patients with Relapsed or Refractory AML1 1Laboratory abnormality is defined as new or worsened by at least one grade from baseline, or if baseline is unknown.
TIBSOVO (500 mg daily)
N=179Parameter All Grades Grade ≥ 3 n (%) n (%) Hemoglobin decreased 108 (60) 83 (46) Sodium decreased 69 (39) 8 (4) Magnesium decreased 68 (38) 0 Uric acid increased 57 (32) 11 (6) Potassium decreased 55 (31) 11 (6) Alkaline phosphatase increased 49 (27) 1 (1) Aspartate aminotransferase increased 49 (27) 1 (1) Phosphate decreased 45 (25) 15 (8) Creatinine increased 42 (23) 2 (1) Alanine aminotransferase increased 26 (15) 2 (1) Bilirubin increased 28 (16) 1 (1) -
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on Ivosidenib
Strong or Moderate CYP3A4 Inhibitors Clinical Impact Prevention or Management - Consider alternative therapies that are not strong or moderate CYP3A4 inhibitors during treatment with TIBSOVO.
- If co-administration of a strong CYP3A4 inhibitor is unavoidable, reduce TIBSOVO to 250 mg once daily [see Dosage and Administration (2.3)].
- Monitor patients for increased risk of QTc interval prolongation [see Warnings and Precautions (5.2)].
Strong CYP3A4 Inducers Clinical Impact - Co-administration of TIBSOVO with strong CYP3A4 inducers decreased ivosidenib plasma concentrations [see Clinical Pharmacology (12.3)].
Prevention or Management - Avoid co-administration of strong CYP3A4 inducers with TIBSOVO.
QTc Prolonging Drugs Clinical Impact - Co-administration of TIBSOVO with QTc prolonging drugs may increase the risk of QTc interval prolongation [see Warnings and Precautions (5.2)].
Prevention or Management - Avoid co-administration of QTc prolonging drugs with TIBSOVO or replace with alternative therapies.
- If co-administration of a QTc prolonging drug is unavoidable, monitor patients for increased risk of QTc interval prolongation [see Warnings and Precautions (5.2)].
7.2 Effect of Ivosidenib on Other Drugs
Ivosidenib induces CYP3A4 and may induce CYP2C9. Co-administration will decrease concentrations of drugs that are sensitive CYP3A4 substrates and may decrease concentrations of drugs that are sensitive CYP2C9 substrates [see Clinical Pharmacology (12.3)]. Use alternative therapies that are not sensitive substrates of CYP3A4 and CYP2C9 during TIBSOVO treatment. Do not administer TIBSOVO with itraconazole or ketoconazole (CYP3A4 substrates) due to expected loss of antifungal efficacy. Co-administration of TIBSOVO may decrease the concentrations of hormonal contraceptives, consider alternative methods of contraception in patients receiving TIBSOVO. If co-administration of TIBSOVO with sensitive CYP3A4 substrates or CYP2C9 substrates is unavoidable, monitor patients for loss of therapeutic effect of these drugs.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal embryo-fetal toxicity studies, TIBSOVO may cause fetal harm when administered to a pregnant woman. There are no available data on TIBSOVO use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In animal embryo-fetal toxicity studies, oral administration of ivosidenib to pregnant rats and rabbits during organogenesis was associated with embryo-fetal mortality and alterations to growth starting at 2 times the steady state clinical exposure based on the AUC at the recommended human dose (see Data). If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Animal Data
Ivosidenib administered to pregnant rats at a dose of 500 mg/kg/day during organogenesis (gestation days 6-17) was associated with adverse embryo-fetal effects including lower fetal weights, and skeletal variations. These effects occurred in rats at approximately 2 times the human exposure at the recommended dose of 500 mg daily.
In pregnant rabbits treated during organogenesis (gestation days 7-20), ivosidenib was maternally toxic at doses of 180 mg/kg/day (exposure approximately 3.9 times the human exposure at the recommended dose of 500 mg daily) and caused spontaneous abortions as well as decreased fetal weights, skeletal variations, and visceral variations.
8.2 Lactation
Risk Summary
There are no data on the presence of ivosidenib or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for adverse reactions in breastfed children, advise women not to breastfeed during treatment with TIBSOVO and for at least 1 month after the last dose.
8.4 Pediatric Use
The safety and effectiveness of TIBSOVO in pediatric patients have not been established.
8.5 Geriatric Use
Thirty-three (97%) of the 34 patients with newly diagnosed AML in the clinical study were 65 years of age or older, and 19 patients (56%) were 75 years or older. One hundred and twelve (63%) of the 179 patients with relapsed or refractory AML in the clinical study were 65 years of age or older and 40 patients (22%) were 75 years or older. No overall differences in effectiveness or safety were observed between patients with relapsed or refractory AML who were 65 years and older and younger patients.
8.6 Renal Impairment
No modification of the starting dose is recommended for patients with mild or moderate renal impairment (eGFR ≥ 30 mL/min/1.73m2, MDRD). The pharmacokinetics and safety of ivosidenib in patients with severe renal impairment (eGFR < 30 mL/min/1.73m2, MDRD) or renal impairment requiring dialysis are unknown [see Clinical Pharmacology (12.3)]. For patients with pre-existing severe renal impairment or who are requiring dialysis, consider the risks and potential benefits before initiating treatment with TIBSOVO.
8.7 Hepatic Impairment
No modification of the starting dose is recommended for patients with mild or moderate (Child-Pugh A or B) hepatic impairment [see Clinical Pharmacology (12.3)]. The pharmacokinetics and safety of ivosidenib in patients with severe hepatic impairment (Child-Pugh C) are unknown. For patients with pre-existing severe hepatic impairment, consider the risks and potential benefits before initiating treatment with TIBSOVO.
-
11 DESCRIPTION
TIBSOVO (ivosidenib) is an inhibitor of isocitrate dehydrogenase 1 (IDH1) enzyme. The chemical name is (2S)-N-{(1S)-1-(2-chlorophenyl)-2-[(3,3-difluorocyclobutyl)-amino]-2-oxoethyl}-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide.
The chemical structure is:
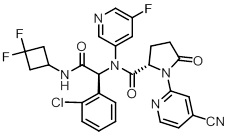
The molecular formula is C28H22C1F3N6O3 and the molecular weight is 583.0 g/mol. Ivosidenib is practically insoluble in aqueous solutions between pH 1.2 and 7.4.
TIBSOVO (ivosidenib) is available as a film-coated 250 mg tablet for oral administration. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet coating includes FD&C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme. Susceptible IDH1 mutations are defined as those leading to increased levels of 2-hydroxyglutarate (2-HG) in the leukemia cells and where efficacy is predicted by 1) clinically meaningful remissions with the recommended dose of ivosidenib and/or 2) inhibition of mutant IDH1 enzymatic activity at concentrations of ivosidenib sustainable at the recommended dosage according to validated methods. The most common of such mutations are R132H and R132C substitutions.
Ivosidenib was shown to inhibit selected IDH1 R132 mutants at much lower concentrations than wild-type IDH1 in vitro. Inhibition of the mutant IDH1 enzyme by ivosidenib led to decreased 2-HG levels and induced myeloid differentiation in vitro and in vivo in mouse xenograft models of IDH1-mutated AML. In blood samples from patients with AML with mutated IDH1, ivosidenib decreased 2-HG levels ex-vivo, reduced blast counts, and increased percentages of mature myeloid cells.
12.2 Pharmacodynamics
Multiple doses of ivosidenib 500 mg daily were observed to decrease plasma 2-HG concentrations in patients with hematological malignancies to levels similar to those observed at baseline in healthy subjects. In bone marrow, 2-HG concentrations were reduced by >90%.
Cardiac Electrophysiology
A concentration-dependent QTc interval prolongation of approximately 17.2 msec (90% CI: 14.7, 19.7) was observed at the steady-state Cmax following a 500 mg daily dose based on an analysis of 171 patients with advanced hematologic malignances and an IDH1 mutation, including 26 patients with newly diagnosed AML and 136 patients with relapsed or refractory AML, who received TIBSOVO 500 mg daily [see Warnings and Precautions (5.1)]. Co-administration with moderate or strong CYP3A inhibitors is expected to further increase QTc interval prolongation from baseline.
12.3 Pharmacokinetics
The following ivosidenib pharmacokinetic parameters were observed following administration of ivosidenib 500 mg as a single dose or daily dose (for steady-state), unless otherwise specified. The steady-state pharmacokinetics of ivosidenib 500 mg were comparable between patients with newly diagnosed AML and patients with relapsed or refractory AML.
The mean peak plasma concentration (Cmax) is 4,503 ng/mL [% coefficient of variation (%CV: 38)] after a single dose, and 6,551 ng/mL (%CV: 44) at steady-state. The steady-state area under the concentration time curve (AUC) is 117,348 ng·hr/mL (%CV: 50).
The AUC and Cmax of ivosidenib increase in a less than dose-proportional manner from 200 mg to 1,200 mg daily (0.4 to 2.4 times the approved recommended dosage). Accumulation ratios were approximately 1.9 for AUC and 1.5 for Cmax over one month. Steady-state plasma levels are reached within 14 days.
Absorption
The median time to Cmax is approximately 3 hours.
Effect of Food
Following administration of a single dose in healthy subjects, a high-fat meal (approximately 900 to 1,000 calories, 500 to 600 fat calories, 250 carbohydrate calories and 150 protein calories) increased ivosidenib Cmax by 98% (90% CI: 79%, 119%) and AUCinf by approximately 25%.
Distribution
The mean apparent volume of distribution of ivosidenib at steady-state is 234 L (%CV: 47). Protein binding of ivosidenib ranges from 92 to 96% in vitro.
Elimination
Ivosidenib has a terminal half-life of 93 hours (%CV: 67) and an apparent clearance (CL/F) of 4.3 L/hour (%CV: 50).
Metabolism
Ivosidenib is the predominant component (>92%) of total radioactivity in plasma. Ivosidenib is primarily metabolized by CYP3A4 with minor contributions by N-dealkylation and hydrolytic pathways.
Excretion
After a single oral administration of radiolabeled ivosidenib to healthy subjects, 77% of ivosidenib was eliminated in the feces (67% as unchanged) and 17% in the urine (10% as unchanged).
Specific Populations
No clinically meaningful effects on the pharmacokinetics of ivosidenib were observed based on age (18 years to 89 years), sex, race (White, Asian, Black or African American), body weight (38 to 150 kg), ECOG performance status, or mild or moderate renal impairment (eGFR ≥30 mL/min/1.73m2, MDRD). The pharmacokinetics of ivosidenib in patients with severe renal impairment (eGFR <30 mL/min/1.73m2, MDRD) or renal impairment requiring dialysis is unknown.
Patients with Hepatic Impairment
Following a single dose of TIBSOVO 500 mg, the geometric mean ratio (90% confidence interval) of ivosidenib systemic exposure (AUC0-INF) in subjects with mild hepatic impairment (Child-Pugh A) was 0.85 (0.62, 1.15) and moderate hepatic impairment (Child-Pugh B) was 0.71 (0.48, 1.05) as compared to that in subjects with normal hepatic function. The pharmacokinetics of ivosidenib in patients with severe hepatic impairment (Child-Pugh C) is unknown.
Drug Interaction Studies
Clinical Studies and Model-Based Approaches
Effect of Strong or Moderate CYP3A4 Inhibitors on Ivosidenib
Itraconazole was used as a strong CYP3A4 index inhibitor to evaluate the effect of CYP3A4 inhibition on the pharmacokinetics of ivosidenib single-dose in a drug-drug interaction study in healthy subjects. Co-administration of 250 mg ivosidenib with itraconazole (200 mg itraconazole once daily for 18 days) increased ivosidenib single-dose AUC to 269% of control (90% CI: 245%, 295%) with no change in Cmax. In regards to multiple-dosing, note that because ivosidenib induces the metabolism of CYP3A4 substrates following ivosidenib multiple dosing, itraconazole (a CYP3A4 substrate) is not recommended to be used concomitantly with TIBSOVO in patients (see Effect of Ivosidenib on CYP3A4 Substrates).
Based on physiologically-based pharmacokinetic modeling, co-administration of 500 mg ivosidenib with the moderate CYP3A4 inhibitor fluconazole (dosed to steady-state) is predicted to increase ivosidenib single-dose AUC to 173% of control with no change in Cmax. In regards to multiple-dosing, co-administration with ivosidenib and fluconazole is predicted to increase ivosidenib steady-state Cmax to 152% of control and AUC to 190% of control [see Drug Interactions (7.1)].
Effect of Strong CYP3A4 Inducers on Ivosidenib
Co-administration of ivosidenib with a strong CYP3A4 inducer (600 mg rifampin once daily for 15 days) is predicted to decrease ivosidenib steady-state AUC by 33% [see Drug Interactions (7.1)].
Effect of Ivosidenib on CYP3A4 Substrates
Ivosidenib induces CYP3A4, including its own metabolism. Co-administration of ivosidenib with CYP3A4 substrates such as itraconazole is expected to decrease itraconazole steady-state AUC to a clinically relevant extent [see Drug Interactions (7.2)].
Effect of Gastric Acid Reducing Agents on Ivosidenib
Gastric acid reducing agents (e.g., proton pump inhibitors, H2-receptor antagonists, antacids) do not affect ivosidenib concentrations.
In vitro Studies
Metabolic Pathways
Ivosidenib may induce CYP2B6, CYP2C8, and CYP2C9 and therefore may affect the pharmacokinetics of sensitive substrates of these enzymes [see Drug Interactions (7.2)].
Drug Transporter Systems
Ivosidenib is a substrate for P-glycoprotein (P-gp). Ivosidenib is not a substrate for BCRP or hepatic transporters OATP1B1 and OATP1B3.
Ivosidenib does not inhibit BCRP, OATP1B1, OATP1B3, OAT1, and OCT2 at clinically relevant concentrations. Ivosidenib is an inhibitor of OAT3 and P-gp.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with ivosidenib. Ivosidenib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. Ivosidenib was not clastogenic in an in vitro human lymphocyte micronucleus assay, or in an in vivo rat bone marrow micronucleus assay. Fertility studies in animals have not been conducted with ivosidenib. In repeat-dose toxicity studies up to 90 days in duration with twice daily oral administration of ivosidenib in rats, uterine atrophy was reported in females at non-tolerated dose levels.
-
14 CLINICAL STUDIES
14.1 Newly-Diagnosed AML
The efficacy of TIBSOVO was evaluated in an open-label, single-arm, multicenter clinical trial (Study AG120-C-001, NCT02074839) that included 28 adult patients with newly-diagnosed AML with an IDH1 mutation. IDH1 mutations were identified by a local or central diagnostic test and confirmed retrospectively using the Abbott RealTime™ IDH1 Assay. The cohort included patients who were age 75 years or older or who had comorbidities that precluded the use of intensive induction chemotherapy based on at least one of the following criteria: baseline Eastern Cooperative Oncology Group (ECOG) performance status of ≥ 2, severe cardiac or pulmonary disease, hepatic impairment with bilirubin > 1.5 times the upper limit of normal, or creatinine clearance < 45 mL/min. TIBSOVO was given orally at a starting dose of 500 mg daily until disease progression, development of unacceptable toxicity, or undergoing hematopoietic stem cell transplantation. Two (7%) of the 28 patients went on to stem cell transplantation following TIBSOVO treatment.
The baseline demographic and disease characteristics are shown in Table 6.
Table 6: Baseline Demographic and Disease Characteristics in Patients with Newly-Diagnosed AML (Study AG120-C-001) ECOG PS: Eastern Cooperative Oncology Group Performance Status. ELN: European Leukemia Net
1 Using confirmatory Abbott RealTime IDH1 assay testing results.
2 Patients were defined as transfusion dependent at baseline if they received any transfusion occurring within 56 days prior to the first dose of TIBSOVO.
3 AML with myelodysplasia-related changes.
Demographic and Disease Characteristics TIBSOVO (500 mg daily)
N=28Demographics Age (Years) Median (Min, Max) 77 (64, 87) Age Categories, n (%) <65 years 1 (4) ≥65 years to <75 years 8 (29) ≥75 years 19 (68) Sex, n (%) Male 15 (54) Female 13 (46) Race, n (%) White 24 (86) Black or African American 2 (7) Asian 0 Native Hawaiian/Other Pacific Islander 0 Other/Not Provided 2 (7) Disease Characteristics ECOG PS, n (%) 0 6 (21) 1 16 (57) 2 5 (18) 3 1 (4) IDH1 Mutation, n (%)1 R132C 24 (86) R132G 1 (4) R132H 2 (7) R132L 1 (4) R132S 0 ELN Risk Category, n (%) Favorable 0 Intermediate 9 (32) Adverse 19 (68) Transfusion Dependent at Baseline2, n (%) 17 (61) Type of AML, n (%) De novo AML 6 (21) AML-MRC3 19 (68) Therapy-related AML 3 (11) Prior Hypomethylating Agent for Antecedent Hematologic Disorder 13 (46) Efficacy was established on the basis of the rate of complete remission (CR) or complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in Table 7. The median follow-up was 8.1 months (range, 0.6 to 40.9 months) and median treatment duration was 4.3 months (range, 0.3 to 40.9 months).
Table 7: Efficacy Results in Patients with Newly-Diagnosed AML (Study AG120-C-001) CI: confidence interval, NE: not estimable
1 CR (complete remission) was defined as <5% blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets >100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter).
2 DOCR (duration of CR), DOCRh (duration of CRh), and DOCR+CRh (duration of CR+CRh) was defined as time since first response of CR, CRh or CR/CRh, respectively, to relapse or death, whichever is earlier. + indicates censored observation.
3 The median durations of CR and CR+CRh were not estimable, with 5 patients (41.7%) who achieved CR or CRh remaining on TIBSOVO treatment (treatment duration range: 20.3 to 40.9 months).
4 CRh (complete remission with partial hematological recovery) was defined as <5% blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets >50,000/microliter and ANC >500/microliter).
Endpoint TIBSOVO (500 mg daily)
N=28CR1 n (%) 8 (28.6) 95% CI (13.2, 48.7) Median DOCR2 (months) NE3 95% CI (4.2, NE) CRh4 n (%) 4 (14.3) 95% CI (4.0, 32.7) Observed DOCRh2 (months) (2.8, 4.6, 8.3, 15.7+) CR+CRh n (%) 12 (42.9) 95% CI (24.5, 62.8) Median DOCR+CRh2 (months) NE3 95% CI (4.2, NE) For patients who achieved a CR or CRh, the median time to CR or CRh was 2.8 months (range, 1.9 to 12.9 months). Of the 12 patients who achieved a best response of CR or CRh, 11 (92%) achieved a first response of CR or CRh within 6 months of initiating TIBSOVO.
Among the 17 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 7 (41.2%) became independent of RBC and platelet transfusions during any 56-day post-baseline period. Of the 11 patients who were independent of both RBC and platelet transfusions at baseline, 6 (54.5%) remained transfusion independent during any 56-day post-baseline period.
14.2 Relapsed or Refractory AML
The efficacy of TIBSOVO was evaluated in an open-label, single-arm, multicenter clinical trial (Study AG120-C-001, NCT02074839) of 174 adult patients with relapsed or refractory AML with an IDH1 mutation. IDH1 mutations were identified by a local or central diagnostic test and confirmed retrospectively using the Abbott RealTime™ IDH1 Assay. TIBSOVO was given orally at a starting dose of 500 mg daily until disease progression, development of unacceptable toxicity, or undergoing hematopoietic stem cell transplantation. Twenty-one (12%) of the 174 patients went on to stem cell transplantation following TIBSOVO treatment.
The baseline demographic and disease characteristics are shown in Table 8.
Table 8: Baseline Demographic and Disease Characteristics in Patients with Relapsed or Refractory AML (Study AG120-C-001) ECOG PS: Eastern Cooperative Oncology Group Performance Status.
1 Using confirmatory Abbott RealTime IDH1 assay testing results.
2 Patients were defined as transfusion dependent at baseline if they received any transfusion occurring within 56 days prior to the first dose of TIBSOVO.
Demographic and Disease Characteristics TIBSOVO (500 mg daily)
N=174Demographics Age (Years) Median (Min, Max) 67 (18, 87) Age Categories <65 years 63 (36) ≥65 years to <75 years 71 (41) ≤75 years 40 (23) Sex, n (%) Male 88 (51) Female 86 (49) Race, n (%) White 108 (62) Black or African American 10 (6) Asian 6 (3) Native Hawaiian/Other Pacific Islander 1 (1) Other/Not provided 49 (28) Disease Characteristics ECOG PS, n (%) 0 36 (21) 1 97 (56) 2 39 (22) 3 2 (1) IDH1 Mutation, n (%)1 R132C 102 (59) R132H 43 (25) R132G 12 (7) R132S 10 (6) R132L 7 (4) Cytogenetic Risk Status, n (%) Intermediate 104 (60) Poor 47 (27) Missing/Unknown 23 (13) Relapse Type Primary refractory 64 (37) Refractory relapse 45 (26) Untreated relapse 65 (37) Relapse Number 0 64 (37) 1 83 (48) 2 21 (12) ≥3 6 (3) Prior Stem Cell Transplantation for AML, n (%) 40 (23) Transfusion Dependent at Baseline2, n (%) 110 (63) Median Number of Prior Therapies (Min, Max) 2 (1, 6) Type of AML, n (%) De novo AML 116 (67) Secondary AML 58 (33) Efficacy was established on the basis of the rate of complete remission (CR) plus complete remission with partial hematologic recovery (CRh), the duration of CR+CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in Table 9. The median follow-up was 8.3 months (range, 0.2 to 39.5 months) and median treatment duration was 4.1 months (range, 0.1 to 39.5 months).
Table 9: Efficacy Results in Patients with Relapsed or Refractory AML (Study AG120-C-001) CI: confidence interval
1 CR (complete remission) was defined as <5% blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets >100,000/microliter and absolute neutrophil counts [ANC] >1,000/microliter).
2 DOCR (duration of CR), DOCRh (duration of CRh), and DOCR+CRh (duration of CR+CRh) was defined as time since first response of CR, CRh or CR/CRh, respectively, to relapse or death, whichever is earlier.
3 CRh (complete remission with partial hematological recovery) was defined as <5% blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets >50,000/microliter and ANC >500/microliter).
4 CR+CRh rate appeared to be consistent across all baseline demographic and baseline disease characteristics with the exception of number of prior regimens.
Endpoint TIBSOVO (500 mg daily)
N=174CR1 n (%) 43 (24.7) 95% CI (18.5, 31.8) Median DOCR2 (months) 10.1 95% CI (6.5, 22.2) CRh3 n (%) 14 (8.0) 95% CI (4.5, 13.1) Median DOCRh2 (months) 3.6 95% CI (1, 5.5) CR+CRh4 n (%) 57 (32.8) 95% CI (25.8, 40.3) Median DOCR+CRh2 (months) 8.2 95% CI (5.6, 12) For patients who achieved a CR or CRh, the median time to CR or CRh was 2 months (range, 0.9 to 5.6 months). Of the 57 patients who achieved a best response of CR or CRh, all achieved a first response of CR or CRh within 6 months of initiating TIBSOVO.
Among the 110 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 41 (37.3%) became independent of RBC and platelet transfusions during any 56-day post-baseline period. Of the 64 patients who were independent of both RBC and platelet transfusions at baseline, 38 (59.4%) remained transfusion independent during any 56-day post-baseline period.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
250 mg tablet: Blue oval-shaped film-coated tablet debossed “IVO” on one side and “250” on the other side.
- 60-count bottles of 250 mg tablets with a desiccant canister (NDC: 71334-100-01)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Differentiation Syndrome
Advise patients of the risks of developing differentiation syndrome as early as 1 day after start of therapy and during the first 3 months on treatment. Ask patients to immediately report any symptoms suggestive of differentiation syndrome, such as fever, cough or difficulty breathing, rash, decreased urinary output, low blood pressure, rapid weight gain, or swelling of their arms or legs, to their healthcare provider for further evaluation [see Boxed Warning and Warnings and Precautions (5.1)].
QTc Interval Prolongation
Inform patients of symptoms that may be indicative of significant QTc interval prolongation including dizziness, lightheadedness, and fainting. Advise patients to report these symptoms and the use of all medications to their healthcare provider [see Warnings and Precautions (5.2)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, including over-the-counter medications, vitamins, and herbal products [see Drug Interactions (7)].
Guillain-Barré Syndrome
Inform patients of symptoms that may be indicative of Guillain-Barré syndrome, including new signs or symptoms of motor and/or sensory neuropathy, such as weakness or tingling sensation in the legs, arms, or upper body, numbness and pain on one side or both sides of the body, changes to any sensory function, or burning or prickling sensation, or difficulty breathing. Advise patients to report these symptoms to their healthcare provider [see Warnings and Precautions (5.3)].
Tumor Lysis Syndrome
Advise patients on the risks of developing tumor lysis syndrome. Advise patients on the importance of maintaining high fluid intake, and the need for frequent monitoring of blood chemistry values [see Adverse Reactions (6.1)].
Gastrointestinal Adverse Reactions
Advise patients on the risks of experiencing gastrointestinal reactions such as diarrhea, nausea, mucositis, constipation, vomiting, decreased appetite and abdominal pain. Ask patients to report these events to their healthcare provider, and advise patients how to manage them [see Adverse Reactions (6.1)].
Lactation
Advise women not to breastfeed during treatment with TIBSOVO and for at least 1 month after the final dose [see Use in Specific Populations (8.2)].
Dosing and Storage Instructions
- Advise patients to swallow tablets whole and to not split, crush, or chew TIBSOVO tablets.
- Advise patients to avoid taking TIBSOVO with a high-fat meal.
- Instruct patients that if a dose of TIBSOVO is vomited, not to take an additional dose, and wait until the next scheduled dose is due. If a dose of TIBSOVO is missed or not taken at the usual time, instruct patients to take the dose as soon as possible unless the next dose is due within 12 hours. Patients can return to the normal schedule the following day.
- Store TIBSOVO at room temperature from 20°C to 25°C (68°F to 77°F).
Manufactured for and marketed by:
Agios Pharmaceuticals, Inc.
Cambridge, MA 02139TIBSOVO® is a registered trademark of Agios Pharmaceuticals, Inc.
© 2018-2019 Agios Pharmaceuticals, Inc.AG-PI-002
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: MAY 2019
MEDICATION GUIDE
TIBSOVO® (tib-SOH-voh)
(ivosidenib) tabletsWhat is the most important information I should know about TIBSOVO?
TIBSOVO may cause serious side effects, including:
-
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death if not treated. Differentiation syndrome has happened as early as 1 day and up to 3 months after starting TIBSOVO. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome while taking TIBSOVO:
- fever
- cough
- trouble breathing
- rash
- decreased urination
- dizziness or lightheadedness
- rapid weight gain
- swelling of your arms or legs
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
See “What are the possible side effects of TIBSOVO?” for more information about side effects.
What is TIBSOVO?
TIBSOVO is a prescription medicine used to treat acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation in:
- adults with newly diagnosed AML who are 75 years or older or who have health problems that prevent the use of certain chemotherapy treatments.
- adults with AML when the disease has come back or has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that TIBSOVO is right for you.
It is not known if TIBSOVO is safe and effective in children.Before taking TIBSOVO, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndrome.
- have problems with abnormal electrolytes, such as sodium, potassium, or magnesium levels.
- have nervous system problems.
- have problems with your kidneys or are on dialysis.
- have any liver disorders, including cirrhosis.
- are pregnant or plan to become pregnant. TIBSOVO may cause harm to your unborn baby. You should avoid becoming pregnant during treatment with TIBSOVO. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with TIBSOVO.
- are breastfeeding or plan to breastfeed. It is not known if TIBSOVO passes into your breast milk. Do not breastfeed during your treatment with TIBSOVO and for at least 1 month after your last dose of TIBSOVO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take hormonal contraceptives. TIBSOVO may affect how hormonal contraceptives work and may cause them to not work as well.
How should I take TIBSOVO?
- Take TIBSOVO exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking TIBSOVO without talking to your healthcare provider.
- Take TIBSOVO 1 time a day about the same time each day.
- Swallow TIBSOVO tablets whole. Do not split, crush, or chew the tablet.
- TIBSOVO can be taken with or without food.
- Do not take TIBSOVO with a high-fat meal. An example of a high-fat meal includes 2 eggs fried in butter, 2 strips of bacon, 2 slices of white bread with butter, 1 croissant with 1 slice of cheese, and 8 ounces of whole milk (approximately 1,000 calories and 58 grams of fat).
- If you vomit after taking a dose of TIBSOVO, do not take an additional dose. Take your next dose at your usual time.
- If you miss a dose of TIBSOVO or did not take it at the usual time, take your dose as soon as possible and at least 12 hours before your next dose. Return to your normal schedule the following day. Do not take 2 doses of TIBSOVO within 12 hours.
What are the possible side effects of TIBSOVO?
TIBSOVO may cause serious side effects, including:
- See “What is the most important information I should know about TIBSOVO?”
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) during treatment with TIBSOVO. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint.
-
Guillain-Barré syndrome has happened in people treated with TIBSOVO. Your healthcare provider will monitor you for nervous system problems and will permanently stop your treatment with TIBSOVO if you develop Guillain-Barré syndrome. Tell your healthcare provider right away if you develop any signs or symptoms of Guillain-Barré syndrome, including:
- weakness or tingling feeling in your legs, arms, or upper body
- numbness and pain on one side or both sides of your body
- any changes in your ability to see, touch, hear, or taste
- burning or prickling sensation
- difficulty breathing
The most common side effects of TIBSOVO include:
- fatigue
- joint pain
- high white blood cell count
- diarrhea
- swelling of arms or legs
- nausea
- shortness of breath
- pain or sores in your mouth or throat
- irregular heart rhythm or heartbeat (QTc prolongation)
- rash
- cough
- decreased appetite
- muscle pain
- constipation
- fever
- hemoglobin decreased (anemia)
- decreased levels of electrolytes in the blood
- changes in liver or kidney function tests
Your healthcare provider will do blood tests before you start and during treatment with TIBSOVO. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with TIBSOVO if you develop side effects.
TIBSOVO may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of TIBSOVO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TIBSOVO?
- Store TIBSOVO at room temperature between 68°C to 77°C (20°C to 25°C).
Keep TIBSOVO and all medicines out of the reach of children.
General information about the safe and effective use of TIBSOVO
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take TIBSOVO for conditions for which it was not prescribed. Do not give TIBSOVO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TIBSOVO that is written for healthcare professionals.
What are the ingredients in TIBSOVO?
Active ingredient: ivosidenib
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet coating includes FD& C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
Manufactured for and marketed by: Agios Pharmaceuticals, Inc. Cambridge, MA 02139
TIBSOVO® is a registered trademark of Agios Pharmaceuticals, Inc.
© 2018-2019 Agios Pharmaceuticals, Inc.
AG-MG-002
For more information go to www.TIBSOVO.com or call 1-833-228-8474.
-
Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death if not treated. Differentiation syndrome has happened as early as 1 day and up to 3 months after starting TIBSOVO. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome while taking TIBSOVO:
- PRINCIPAL DISPLAY PANEL - NDC: 71334-100-01 - 250 mg Tablet 60-Count Bottle Label
- PRINCIPAL DISPLAY PANEL - NDC: 71334-100-02 - 250 mg Tablet 14-Count Bottle Label (Professional Sample)
-
INGREDIENTS AND APPEARANCE
TIBSOVO
ivosidenib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71334-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVOSIDENIB (UNII: Q2PCN8MAM6) (IVOSIDENIB - UNII:Q2PCN8MAM6) IVOSIDENIB 250 mg Product Characteristics Color BLUE Score no score Shape OVAL Size 18mm Flavor Imprint Code IVO;250 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71334-100-01 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211192 07/20/2018 Labeler - Agios Pharmaceuticals, Inc. (011567735)
Trademark Results [Tibsovo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TIBSOVO 87556035 5587521 Live/Registered |
Agios Pharmaceuticals, Inc. 2017-08-04 |
 TIBSOVO 87556017 5587520 Live/Registered |
Agios Pharmaceuticals, Inc. 2017-08-04 |
 TIBSOVO 87122179 5444093 Live/Registered |
Agios Pharmaceuticals, Inc. 2016-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

