Meridian® Unwrapped antibacterial deodorant soap
Meridian Unwrapped antibacterial deodorant by
Drug Labeling and Warnings
Meridian Unwrapped antibacterial deodorant by is a Otc medication manufactured, distributed, or labeled by Bob Barker Company Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MERIDIAN UNWRAPPED ANTIBACTERIAL DEODORANT- benzethonium chloride soap
Bob Barker Company Inc.
----------
Meridian® Unwrapped antibacterial deodorant soap
Inactive Ingredients
Sodium Palmate, Calcium Carbonate, Water, Sodium Palm Kernelate, Talc, Palm Acid, Glycerin, Sodium Chloride, Sodium Laureth Sulfate, Titanium Dioxide, Palm Kernel Acid, Fragrance, Tetrasodium EDTA, Tetrasodium Etidronate
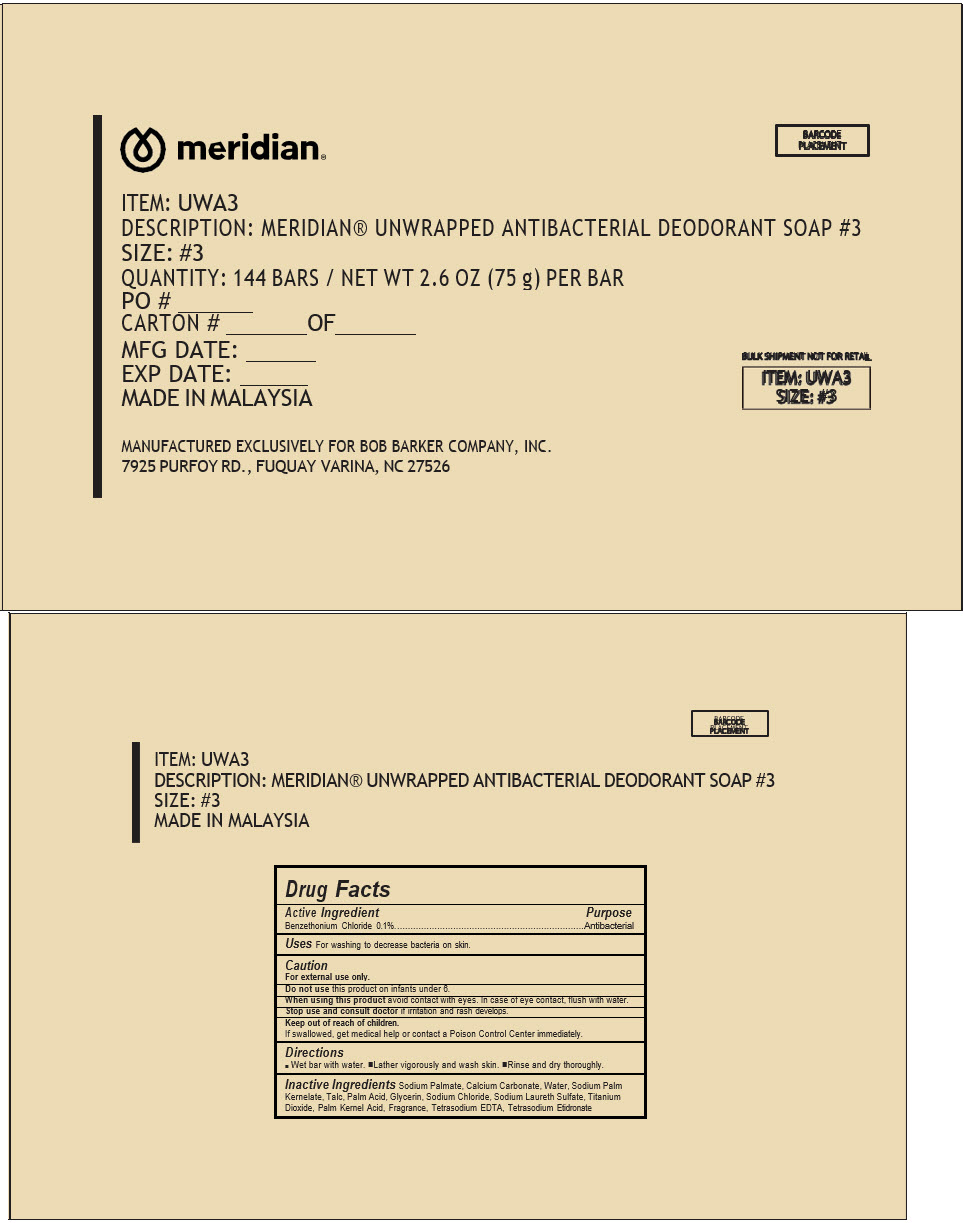
PRINCIPAL DISPLAY PANEL - 144 Bar Case
meridian®
ITEM: UWA3
DESCRIPTION: MERIDIAN® UNWRAPPED ANTIBACTERIAL DEODORANT SOAP #3
SIZE: #3
QUANTITY: 144 BARS / NET WT 2.6 OZ (75 g) PER BAR
PO # _______
CARTON # ________ OF _______
MFG DATE: _______
EXP DATE: ______
MADE IN MALAYSIA
MANUFACTURED EXCLUSIVELY FOR BOB BARKER COMPANY, INC.
7925 PURFOY RD., FUQUAY VARINA, NC 27526
BULK SHIPMENT NOT FOR RETAIL
ITEM: UWA3
SIZE: #3
| MERIDIAN UNWRAPPED ANTIBACTERIAL DEODORANT
benzethonium chloride soap |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bob Barker Company Inc. (058525536) |