G.M. Collin Acne by Laboratoires Dermo Cosmetik Inc
G.M. Collin Acne by
Drug Labeling and Warnings
G.M. Collin Acne by is a Otc medication manufactured, distributed, or labeled by Laboratoires Dermo Cosmetik Inc . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
G.M. COLLIN ACNE- salicylic acid
Laboratoires Dermo Cosmetik Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
Directions
Apply and emulsify carefully with wet fingers. Rinse with lukewarm water. Complete with the adequate G.M. Collin treating lotion. Morning and evening or as recommended by your skin care professional.
Other information
Store in a cool, dry place. Avoid high temperatures. Protect from freezing. Keep away from flame, fire and heat.
Inactive ingredients
WATER/EAU, SODIUM LAURYL GLUCOSE CARBOXYLATE, LAURYL GLUCOSIDE, COCO-BETAINE, GLYCOLIC ACID, COCO-GLUCOSIDE, GLYCERYL OLEATE, PEG-120 METHYL GLUCOSE DIOLEATE, SODIUM CITRATE, SODIUM HYDROXIDE, ALOE BARBADENSIS LEAF JUICE, PELARGONIUM GRAVEOLENS (GERANIUM) OIL, MENTHA ARVENSIS OIL, CYMBOPOGON MARTINI (PALMA ROSA) OIL, CITRUS AURANTIUM DULCIS (ORANGE) OIL, LACTOBACILLUS FERMENT LYSATE FILTRATE, PEUMUS BOLDUS LEAF EXTRACT, BUTYLENE GLYCOL, XANTHAN GUM, CHAMOMILLA RECUTITA (MATRICARIA) FLOWER EXTRACT, ARCTIUM MAJUS ROOT (BURDOCK) EXTRACT, ARNICA MONTANA FLOWER EXTRACT, URTICA DIOICA (NETTLE) EXTRACT, EQUISETIUM ARVENSE (HORSETAIL) EXTRACT, BETULA ALBA LEAF EXTRACT, SALVIA OFFICINALIS (SAGE) LEAF EXTRACT
INGREDIENTS
WATER/EAU, CITRUS AURANTIUM AMARA (BITTER ORANGE) FLOWER WATER, C12-15 ALKYL BENZOATE, PEG-40 CASTOR OIL, CITRUS LIMONUM (LEMON) PEEL OIL, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF OIL, CYMBOPOGON NARDUS (CITRONELLA) OIL, MENTHA ARVENSIS LEAF OIL, ABIES SIBIRICA OIL, CINNAMOMUM CAMPHORA OIL, ANIBA ROSAEODORA (ROSEWOOD) WOOD OIL, EUGENIA CARYOPHYLLUS OIL, SODIUM BENZOATE, ALOE BARBADENSIS LEAF JUICE, POTASSIUM SORBATE, SALICYLIC ACID, POTASSIUM ALUM, CITRIC ACID, YELLOW 5, BLUE 1
Warnings
For external use only.
Directions
After the G.M. Collin cleanser and treating lotion, apply the quantity of gel suited for individual skin absorption. Morning and/or evening or as recommended by your skin care professional.
Other information
Store in a cool, dry place. Avoid high temperatures. Protect from freezing. Keep away from flame, fire and heat.
Inactive ingredients
WATER/EAU, AMMONIUM HYDROXIDE, SODIUM HYALURONATE, HYDROLYZED GLYCOSAMINOGLYCANS, BUTYLENE GLYCOL, PEG-8, ETHOXYDIGLYCOL, POLY (METHYL VINYL ETHER/MALEIC ANHYDRE) DECADIENE CROSSPOLYMER, DIMETHICONE PEG-7 PHOSPHATE, PEG-60 ALMOND GLYCERIDES, CAPRYLYL GLYCOL, GLYCERIN, CARBOMER, NORDIHYDROGUAIARETIC ACID, OLEANOLIC ACID, PEG-40 HYDROGENATED CASTOR OIL, PANTHENOL, MANNITOL, YEAST EXTRACT/EXTRAIT DE LEVURE, GLYCOGEN, MAGNESIUM ASCORBYL PHOSPHATE (OXYGEN COMPLEX), PELARGONIUM GRAVEOLENS (GERANIUM) OIL, MENTHA ARVENSIS OIL, CYMBOPOGON MARTINI (PALMA ROSA) OIL, CITRUS AURANTIUM DULCIS (ORANGE) OIL, POLYSORBATE 20, PALMITOYL OLIGOPEPTIDE, PALMITOYL TETRAPEPTIDE-7, LACTOBACILLUS FERMENT LYSATE FILTRATE, ZINC ACETYLMETHIONATE, PEUMUS BOLDUS LEAF EXTRACT, XANTHAN GUM, MAGNESIUM ASPARTATE, ZINC GLUCONATE, COPPER GLUCONATE, ALOE BARBADENSIS LEAF JUICE, ALLANTOIN, POTASSIUM ASCORBYL TOCOPHERYL PHOSPHATE (VITAMIN C, VITAMIN E), SODIUM COPPER CHLOROPHYLLIN
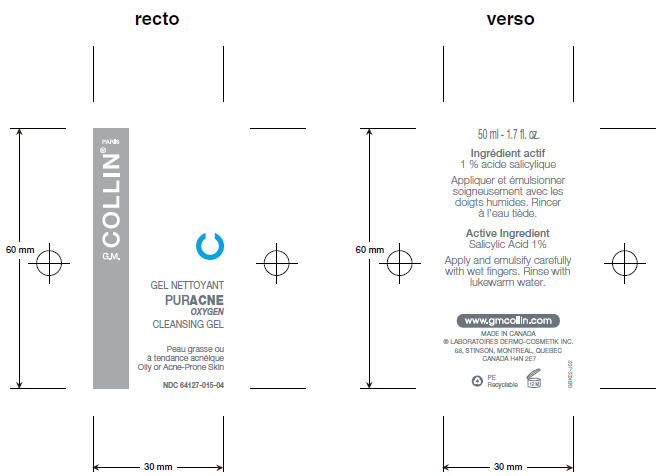
PRINCIPAL DISPLAY PANEL - 50 mL Bottle
GEL NETTOYANT
PURACNE
OXYGEN
CLEANSING GEL
Oily or Acne-Prone Skin
NDC: 64127-015-04
PARIS
COLLIN®
G.M.
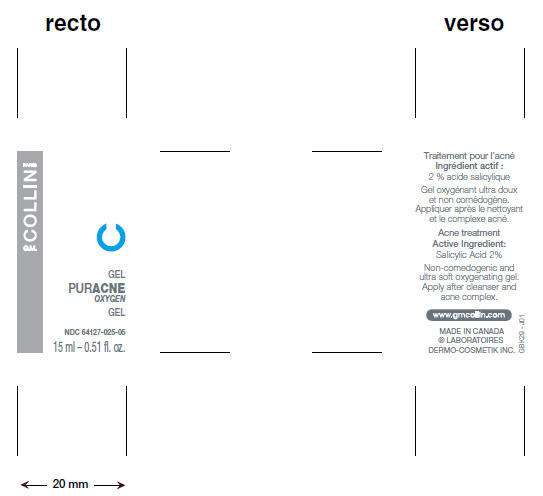
PRINCIPAL DISPLAY PANEL - 15 mL Bottle
GEL
PURACNE
OXYGEN
GEL
NDC: 64127-025-05
15 ml – 0.51 fl. oz.
PARIS
COLLIN
G.M.
PRINCIPAL DISPLAY PANEL - Carton
GEL
Oily or Acne-Prone Skin
NDC: 64127-075-02
PURACNE OXYGEN
CLEANSING GEL
NDC: 64127-015-04
50 ml – 1.7 fl. oz.
ACNE
COMPLEX
15 ml – 0.51 fl. oz.
PURACNE OXYGEN
GEL
NDC: 64127-025-05
15 ml - 0.51 fl. oz.
PARIS
COLLIN®
G.M.

| G.M. COLLIN ACNE
salicylic acid kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Laboratoires Dermo Cosmetik Inc (249335480) |