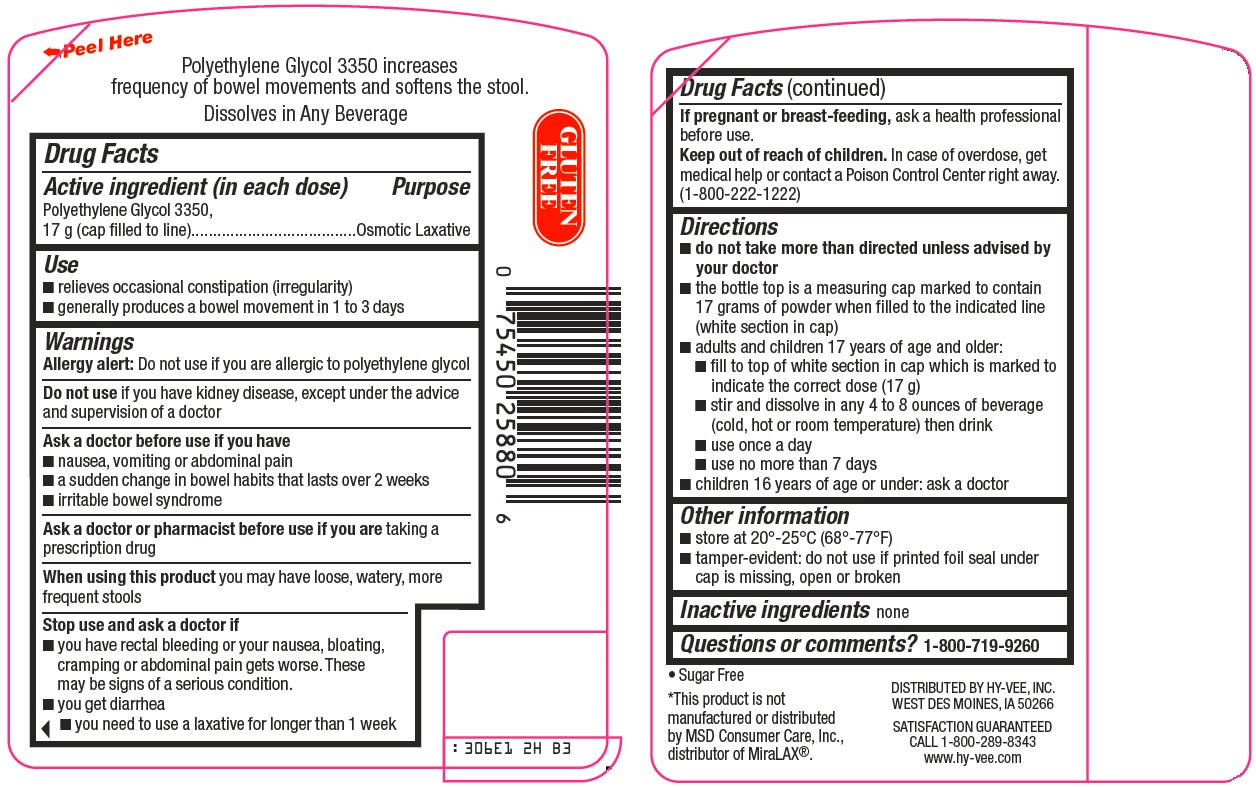
Hy-Vee, Inc. ClearLax® Drug Facts
clear lax by
Drug Labeling and Warnings
clear lax by is a Otc medication manufactured, distributed, or labeled by HyVee Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CLEAR LAX ORIGINAL PRESCRIPTION STRENGTH- polyethylene glycol 3350 powder, for solution
HyVee Inc
----------
Hy-Vee, Inc. ClearLax® Drug Facts
Use
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 1 to 3 days
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
Directions
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or under: ask a doctor
| CLEAR LAX
ORIGINAL PRESCRIPTION STRENGTH
polyethylene glycol 3350 powder, for solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - HyVee Inc (006925671) |
Revised: 12/2018
Document Id: 5308c4f7-5721-4014-ac7f-94bcac38192b
Set id: 5bcf01d8-ddf3-4e64-8d3f-3526a51a5859
Version: 5
Effective Time: 20181210
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.