EXTRANEAL- icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution
EXTRANEAL by
Drug Labeling and Warnings
EXTRANEAL by is a Prescription medication manufactured, distributed, or labeled by Vantive US Healthcare LLC, Vantive Manufacturing Pte. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
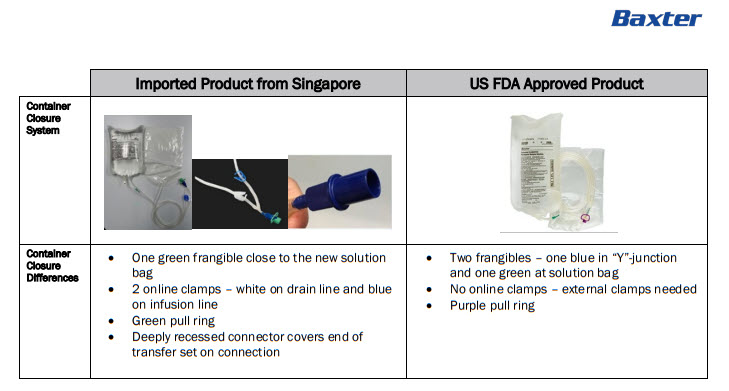
- Health Care Provider Letter
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FNB4984
2000 mL
(APPROX 80 mL EXCESS)BaxterLogo
EXTRANEAL Peritoneal Dialysis Solution with 7.5% Icodextrin
EACH 100 mL CONTAINS
7.5 G ICODEXTRIN 538 mg SODIUM CHLORIDE USP 448 mg SODIUM LACTATE
25.7 mg CALCIUM CHLORIDE USP 5.08 mg MAGNESIUM CHLORIDE USP pH 5.2 (5.0 to 6.0)
mmol/LSODIUM 132 CALCIUM 1.75 MAGNESIUM 0.25 CHLORIDE 96 LACTATE 40
OSMOLARITY284 mOsmol/L (CALC)
STERILE NONPYROGENIC STORE BELOW 30°C SEE INSERT
FOR INRAPERITONEAL ADMINISTRATION ONLY NOT FOR INTRAVENOUS USE
CAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION KEEP OUT OF REACH OF CHILDRENMANUFACTURED BY
BAXTER HEALTHCARE SA, SINGAPORE BRANCH
2 WOODLANDS INDUSTRIAL PARK D STREET 2 SINGAPORE 737778
(AN AFFILIATE OF BAXTER HEALTHCARE CORPORATION USA)
DIRECTION TO BE USED AS DIRECTED BY PHYSICIAN
FOR HONG KONG ONLY: PRESCRIPTION DRUG
IMPORTED BY BAXTER HEALTHCARE (THAILAND) CO., LTD., BANGKOK
FOR INDONESIA ONLY: HARUS DENGAN RESEP DOKTER
IMPORTED & MARKETED BY PT KALBE FARMA TBK, BEKSAI – INDONESIA
FOR MALAYSIA ONLY: CONTROLLED MEDICINE. JAUHI DARIPADA KANAK-KANAK
PRODUCT REGISTRATION HOLDER: BAXTER HEALTHCARE (MALAYSIA) SDN. BHD.,
B-21-3A, THE ASCENT, PARADIGM, 1, JLN SS7/26A, 47301 PJ SELANGOR, MALAYSIAULTRABAGCONTAINER PL-146
SIN11220P MAL06021264AZ
HK-46627 BRU15011744P
INDONESIA DKI 0915600242A1
THAI REG. NO. 2C 16/51 (N)30
023687
2 95 28
EXTRANEAL, UL-TRABAG and PL-146are trademarks of Baxter International Inc.
3A-00004
FNB4984SG
barcode
Lot:
barcode7.5% ICODEXTRIN
EXTRANEAL
6 X 2000 ML
ULTRABAG MAL 06021264AZ023687
Barcode
18806466008418LOT: S24A12345
EXP: 12.12.2024 -
INGREDIENTS AND APPEARANCE
EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0707 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICODEXTRIN (UNII: 2NX48Z0A9G) (ICODEXTRIN - UNII:2NX48Z0A9G) ICODEXTRIN 7.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 25.7 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0707-06 6 in 1 CARTON 10/23/2024 02/12/2027 1 NDC: 0941-0707-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/23/2024 02/12/2027 Labeler - Vantive US Healthcare LLC (119181963) Establishment Name Address ID/FEI Business Operations Vantive Manufacturing Pte. Ltd. 599464843 analysis(0941-0707) , label(0941-0707) , manufacture(0941-0707) , pack(0941-0707) , sterilize(0941-0707)
Trademark Results [EXTRANEAL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXTRANEAL 78566260 not registered Dead/Abandoned |
Baxter International Inc. 2005-02-13 |
 EXTRANEAL 76221877 not registered Dead/Abandoned |
Baxter International Inc. 2001-03-09 |
 EXTRANEAL 76221144 2843444 Live/Registered |
Baxter International Inc. 2001-03-08 |
 EXTRANEAL 75265684 not registered Dead/Abandoned |
Baxter International Inc. 1997-03-28 |
 EXTRANEAL 75250390 not registered Dead/Abandoned |
Baxter International Inc. 1997-03-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.