AMPROL 25- amprolium powder
Amprol 25 by
Drug Labeling and Warnings
Amprol 25 by is a Animal medication manufactured, distributed, or labeled by Huvepharma, Inc., Huvepharma EOOD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BAG FRONT
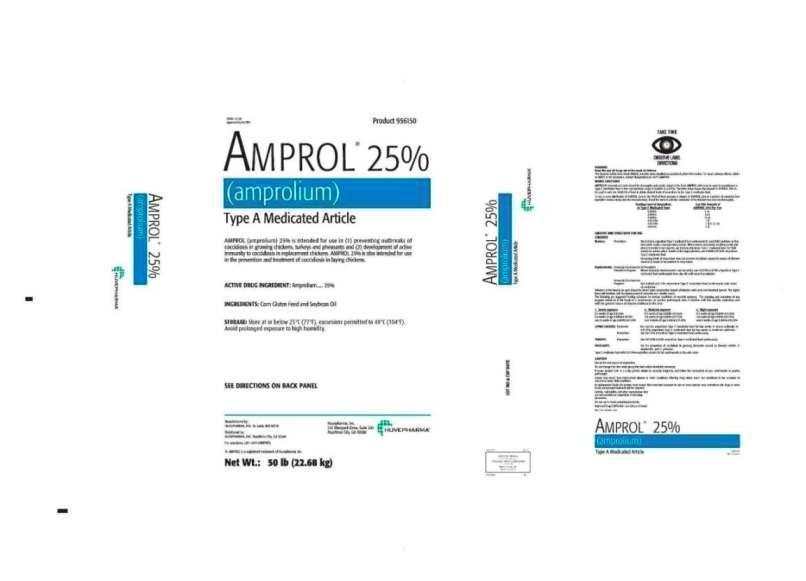
NADA 12-350 Product 956150
Approved by FDA
AMPROL®25%
(amprolium)
Type A Medicated ArticleAMPROL (amprolium) 25% is intended for use in (1) preventing outbreaks
of coccidiosis in growing chickens, turkeys and pheasants and (2) development
of active immunity to coccidiosis in replacement chickens. AMPROL 25% is also
intended for use in the prevention and treatment of coccidiosis in laying chickens.
ACTIVE DRUG INGREDIENT: Amprolium ......25%
INGREDIENTS: Corn Gluten Feed and Soybean OilSTORAGE: Store at or below 25°C (77°F), excursions permitted to 40°C (104°F).
Avoid prolonged exposure to high humidity.
SEE DIRECTIONS ON BACK PANEL
Manufactured by: Huvepharma, Inc.
Huvepharma, Inc., St. Louis, MO 63116 525 Westpark Drive, Suite 230
Peachtree City, GA 30269
Distributed by:
Huvepharma, Inc., Peachtree City, GA 30269
For questions, call 1-877-4AMPROL
®AMPROL is a registered trademark of Huvepharma, Inc.
Net Wt.: 50 lb (22.68 kg) - WARNING
-
MIXING DIRECTIONS
AMPROL (amprolium) 25% should be thoroughly and evenly mixed in the feed. AMPROL 25% may be used to manufacture a Type C medicated feed in the concentration range 0f 0.004% to 0.025%. The table below shows the amount of AMPROL 25% to be used in each ton (2000 lb) of feed to obtain desired levels of amprolium in the Type C medicated feed.
To aid in even distribution of AMPROL 25% in the finished feed, prepare a mixture of AMPROL 25% in a portion of complete feed ingredient before mixing into the finished ration. Blend the mixture with the remainder of the finished feed and mix thoroughly.Feeding level of Amprolium
In Type C Medicated Feeds
Use This Amount of
AMPROL 25% Per Ton
0.004%
5 oz
0.006%
8 oz
0.008%
10 oz
0.0125%
1 lb
0.0175%
1 lb 6 ½ oz
0.025%
2 lb
AMOUNT AND DIRECTIONS FOR USE
CHICKENS
Broilers
Prevention
Use 0.0125% amprolium Type C medicated feed continuously for most field conditions as they exist under modern management practices. Where severe coccidiosis conditions exists and where immunity is not required, use 0.025% amprolium Type C medicated feed. For field conditions where only E. tenella is the major problem, use 0.008%-0.0125% amprolium Type C medicated feed.
Increasing levels of amprolium may not prevent coccidiosis caused by strains of Eimeria commonly found to be resistant to Amprolium.
Replacements:
Immunity Development or Prevention
Prevention Program:
Where immunity development is not desirable, use 0.0125% -0.025% amprolium Type C medicated feed continuously from day old until onset of production
Immunity Development
Program:
Use 0.004%-0.0125%ampfolium Type C medicated feed continuously until onset of production
Selection of the level to be used should be based upon comparative hazard of infection with cecal and intestinal species. The higher levels will interfere with the development immunity to E. tenella (cecal).
The following are suggested feeding schedules for various conditions of coccidial exposure. The planning and evaluation of any program should be in the hands of a veterinarian or poultry pathologist who is familiar with the specific operation and with the general nature of disease problems in the area.
1. Severe exposure
2. Moderate exposure
3. Slight exposure
0-5 weeks of age 0.0125%
0-5 weeks of age 0.008%-0.0125%
0-5 weeks of age 0.004%-0.0125%
5-8 weeks of age 0.008%-0.0125%
5-8 weeks of age 0.006%-0.0125%
5-8 weeks of age 0.004%-0.0125%
over 8 weeks of age 0.004%-0.0125%
over 8 weeks of age 0.004%-0.0125%
over 8 weeks of age 0.004%-0.0125%
LAYING CHICKENS:
Treatment:
Use 0.025% amprolium Type C medicated feed for two weeks in severe outbreaks or 0.0125% amprolium Type C medicated feed for two weeks in moderate outbreaks.
Prevention
Use 0.0125% amprolium Type C medicated feed continuously.
TURKEYS:
Prevention
Use 0.0125%-0.025% amprolium Type C medicated feed continuously.
PHEASANTS:
For the prevention of coccidiosis in growing pheasants caused by Eimeria colchici, E. duodenalis, and E. phasiani.
Type C medicated feed with 0.0175% amprolium should be fed continuously as the sole ration. -
CAUTION:
Use as the sole source of amprolium.
Do not change the litter while giving this feed unless absolutely necessary.
If losses exceed 0.5% in a 2-day period, obtain an accurate diagnosis, and follow the instructions of your veterinarian or poultry pathologist.
Losses may result from intercurrent disease or other conditions affecting drug intake which can contribute to the virulence of coccidiosis under field conditions.
In replacement flocks the grower must expect that excessive exposure to one or more species may overwhelm the drug in some flocks and prompt treatment will be required.
Fertility, hatchability and other reproductive data are not available on amprolium in breeding pheasants.
Do not use in feeds containing bentonite.
Restricted Drug (California) Use Only as Directed. - STORAGE AND HANDLING
- Amprol 25 Bag Label
-
INGREDIENTS AND APPEARANCE
AMPROL 25
amprolium powderProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC: 23243-9561 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMPROLIUM (UNII: 95CO6N199Q) (AMPROLIUM ION - UNII:H2T307KMZR) AMPROLIUM 250 g in 1 kg Product Characteristics Color brown ((Light Tan to Brown)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23243-9561-5 22.68 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA012350 05/29/2009 Labeler - Huvepharma, Inc. (619153559) Registrant - Huvepharma EOOD (552691651) Establishment Name Address ID/FEI Business Operations Huvepharma, Inc. 883128204 medicated animal feed manufacture, analysis, pack, label, manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.