WHITE IS BLACK by CURADEN AG / CURADEN PRODUCTION AG
WHITE IS BLACK by
Drug Labeling and Warnings
WHITE IS BLACK by is a Otc medication manufactured, distributed, or labeled by CURADEN AG, CURADEN PRODUCTION AG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WHITE IS BLACK- sodium monofluorophosphate gel, dentifrice
CURADEN AG
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
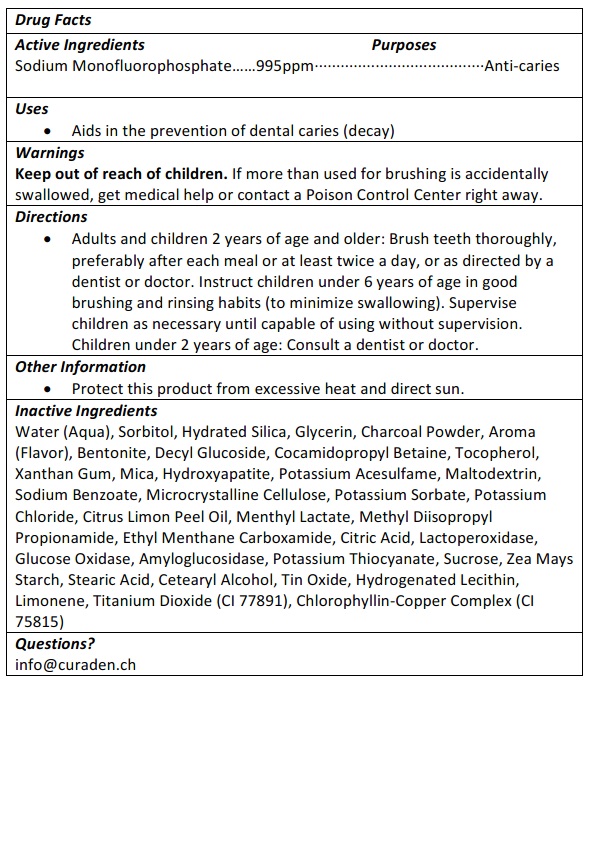
DIRECTIONS
ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER: BRUSH TEETH THOROUGHLY, PREFERABLY AFTER EACH MEAL OR ATLEAST TWICE A DAY, OR AS DIRECTED BY A DENTIST OR DOCTOR. INSTRUCT CHILDREN UNDER 6 YEARS OF AGE IN GOOD BRUSHING AND RINSING HABITS (TO MINIMIZE SWALLOWING). SUPERVISE CHILDREN AS NECESSARY UNTIL CAPABLE OF USING WITHOUT SUPERVISION.
CHILDREN UNDER 2 YEARS OF AGE: CONSULT A DENTIST OR DOCTOR.
WARNINGS: IF MORE THAN THE AMOUNT USED FOR BRUSHING IS ACCIDENTALLY SWALLOWED, GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
INACTIVE INGREDIENTS
Water (Aqua), Sorbitol, Hydrated Silica, Glycerin, Charcoal Powder, Aroma (Flavor), Bentonite, Decyl Glucoside, Cocamidopropyl Betaine, Tocopherol, Xanthan Gum, Mica, Hydroxyapatite, Potassium Acesulfame, Maltodextrin, Sodium Benzoate, Microcrystalline Cellulose, Potassium Sorbate, Potassium Chloride, Citrus Limon Peel Oil, Menthyl Lactate, Methyl Diisopropyl Propionamide, Ethyl Menthane Carboxamide, Citric Acid, Lactoperoxidase, Glucose Oxidase, Amyloglucosidase, Potassium Thiocyanate, Sucrose, Zea Mays Starch, Stearic Acid, Cetearyl Alcohol, Tin Oxide, Hydrogenated Lecithin, Limonene, Titanium Dioxide (CI 77891), Chlorophyllin-Copper Complex (CI 75815)
| WHITE IS BLACK
sodium monofluorophosphate gel, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CURADEN AG (480027255) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CURADEN PRODUCTION AG | 486745818 | manufacture(71112-101) | |