ANASCORP (centruroides (scorpion) immune f(ab)2- equine injection, powder, lyophilized, for solution
ANASCORP by
Drug Labeling and Warnings
ANASCORP by is a Other medication manufactured, distributed, or labeled by Rare Disease Therapeutics, Inc, Instituto Bioclon S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ANASCORP safely and effectively. See full prescribing information for ANASCORP.
ANASCORP®
Centruroides (Scorpion) Immune F(ab')2 (Equine)
Injection
Lyophilized for Solution
For Intravenous Use Only
Initial U.S. Approval: 2011INDICATIONS AND USAGE
- ANASCORP is an antivenin indicated for the treatment of clinical signs of scorpion envenomation. (1)
DOSAGE AND ADMINISTRATION
Intravenous use only.
Initial Dose 3 vials - Reconstitute each vial with 5 milliliters of sterile normal saline (0.9% NaCl).
- Combine and further dilute to a total of 50 milliliters.
- Infuse intravenously over 10 minutes.
Additional dose (s) As needed - Administer one vial at a time at 30-60 minute intervals.
- Dilute to a total of 50 milliliters with sterile normal saline (0.9% NaCl).
- Infuse intravenously over 10 minutes.
- Initiate treatment with ANASCORP as soon as possible after scorpion sting in patients who develop clinically important signs of scorpion envenomation, including but not limited to loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth, vomiting.(2)
- Close patient monitoring is necessary.(2)
DOSAGE FORMS AND STRENGTHS
- Each vial contains a sterile, lyophilized preparation containing not more than 19 milligrams total protein and not less than 150 LD50 (mouse) neutralizing units.(3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Severe hypersensitivity reactions, including anaphylaxis, are possible with ANASCORP. Prepare for monitoring and management of allergic reactions, particularly in patients with a history of hypersensitivity to equine (horse) proteins or patients who have received previous therapy with antivenoms containing scorpion or equine proteins.(5.1)
- Delayed allergic reactions (serum sickness) may occur following treatment with ANASCORP. Patient monitoring with follow-up visit is recommended.(5.2)
- ANASCORP is made from equine plasma and may contain infectious agents, e.g. viruses.(5.3)
- Localized reactions and generalized myalgias have been reported with the use of cresol as an injectable excipient.(5.4)
ADVERSE REACTIONS
The most common adverse reactions observed in ≥ 2% of patients in the clinical studies for ANASCORP were: vomiting, pyrexia, rash, nausea and pruritus.(6)
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1 877-851-1902, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
Pregnancy: No human or animal data. Use only if clearly needed.(8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Delayed Allergic Reactions (Serum Sickness)
5.3 Transmissible Infectious Agents
5.4 Reaction to Cresol
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For Intravenous use only.
Initiate treatment with ANASCORP as soon as possible after scorpion sting in patients who develop clinically important signs of scorpion envenomation, including but not limited to loss of muscle control, roving or abnormal eye movements, slurred speech, respiratory distress, excessive salivation, frothing at the mouth and vomiting.(2)
Initial Dose: 3 vials
- The initial dose of ANASCORP is 3 vials.
- Reconstitute the contents of each vial with 5 milliliters of sterile normal saline (0.9% NaCl) and mix by continuous gentle swirling.
- Combine the contents of the reconstituted vials promptly and further dilute to a total volume of 50 milliliters with sterile normal saline (0.9% NaCl).
- Inspect the solution visually for particulate matter and discoloration prior to administration. Do not use if turbid.
- Infuse intravenously over 10 minutes.
- Monitor patient closely during and up to 60 minutes following the completion of infusion to determine if clinically important signs of envenomation have resolved.
- Discard partially used vials.
Additional Dosing
- Additional doses may be used if needed.
- Infuse one vial at a time at intervals of 30 to 60 minutes.
- Reconstitute the contents with 5 milliliters of sterile normal saline (0.9% NaCl) and mix by continuous gentle swirling.
- Further dilute to a total volume of 50 milliliters with sterile normal saline (0.9% NaCl). Inspect the solution visually for particulate matter or discoloration prior to administration.
- Infuse intravenously over 10 minutes.
- Monitor patient closely during and up to 60 minutes following the completion of infusion to determine if clinically important signs of envenomation have resolved.
- Discard partially used vials.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Severe hypersensitivity reactions, including anaphylaxis, may occur with ANASCORP. Close patient monitoring for hypersensitivity reactions and readiness with intravenous therapy using epinephrine, corticosteroids, and diphenhydramine hydrochloride is recommended during the infusion of ANASCORP. If an anaphylactic reaction occurs during the infusion, terminate administration at once and administer appropriate emergency medical care.
Patients with known allergies to horse protein are particularly at risk for an anaphylactic reaction. Patients who have had previous therapy with ANASCORP or another equine antivenom/antitoxin may have become sensitized to equine protein and be at risk for a severe hypersensitivity reaction.5.2 Delayed Allergic Reactions (Serum Sickness)
Monitor patients with follow-up visit(s) for signs and symptoms of delayed allergic reactions or serum sickness (e.g., rash, fever, myalgia, arthralgia), and treat appropriately if necessary. Eight out of 1,534 (0.5%) patients in the clinical trials exhibited symptoms suggestive of serum sickness. (6.1)
-
6 ADVERSE REACTIONS
The most common adverse reactions observed in ≥ 2% of patients in the clinical studies for ANASCORP were: vomiting, pyrexia, rash, nausea and pruritus.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 1534 patients were treated with ANASCORP, ranging from less than one month to 90 years old. The patient population was comprised of 802 males and 732 females. Patients were monitored for signs and symptoms of adverse reactions, including acute hypersensitivity reactions and serum sickness. Follow-up telephone interviews were conducted at 24 hours, 7 days, and 14 days after treatment to assess symptoms suggestive of ongoing venom effect, serum sickness, and any other adverse reactions.
Table 1 shows the adverse reactions occurring in patients across all clinical trials for ANASCORP. Twenty-seven percent (421/1534) of patients receiving ANASCORP reported at least one adverse reaction.Table 1: Adverse Reactions Reported in ≥ 1% of Patients ADVERSE REACTIONS ANASCORP [N=1534]
n(%)Vomiting 72 (4.7) Pyrexia 63 (4.1) Rash 41 (2.7) Nausea 32 (2.1) Pruritus 31 (2.0) Headache 29 (1.9) Rhinorrhea 28 (1.8) Myalgia 25 (1.6) Fatigue 24 (1.6) Cough 22 (1.4) Diarrhea 20 (1.3) Lethargy 17 (1.1) No patients died or discontinued study participation for severe adverse reactions.
Eight patients were considered to have serum sickness (Type III hypersensitivity); no patient manifested the full serum sickness syndrome. Three patients were treated with systemic corticosteroids and five others received either no treatment or symptomatic therapy.
34 patients experienced a total of 39 severe adverse reactions such as respiratory distress, aspiration, hypoxia, ataxia, pneumonia, and eye swelling. It is not clear whether these adverse reactions were related to ANASCORP envenomation or a combination of both.6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ANASCORP . Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Chest tightness, palpitations, rash and pruritus. - 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with ANASCORP. It is also not known whether ANASCORP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. ANASCORP should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether ANASCORP is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when ANASCORP is administered to a nursing woman.
8.4 Pediatric Use
Seventy-eight percent of the patients enrolled in the clinical studies were pediatrics subjects(1204/1534), with ages ranging from less than one month to 18.7 years of age. Patient age groups were as follows: < 2 years of age, 29%, 2 to 5 years, 37%, 5 to 18 years, 34%. The efficacy and safety of ANASCORP is comparable in pediatric and adult patients.
-
11 DESCRIPTION
ANASCORP [Centruroides (Scorpion) Immune F(ab')2 (Equine) Injection] is a sterile nonpyrogenic, lyophilized, polyvalent preparation of equine immune globulin F(ab')2 fragments, manufactured from plasma of horses immunized with with venom of C. noxius, C.l. limpidus, C.l. tecomanus, and C.s.suffusus. The product is obtained by pepsin digestion of horse plasma to remove the Fc portion of immune globulin, followed by fractionation and purification steps. The F(ab')2 content is not less than 85%, F(ab) content is not more than 7%, and the product contains less than 5% intact immunoglobulin. Each vial of ANASCORP contains 45-80 milligrams of sodium chloride, 4.3 - 38.3 milligrams of sucrose, and 6.6-94.9 milligrams of glycine as stabilizers. Trace amounts of pepsin, cresol (< 0.058 mg/vial), borates (< 1 mg/vial) and Sulfates (< 1.7 mg/vial) may be present from the manufacturing process. Each vial contains no more than 19 milligrams of protein and will neutralize at least 150 LD50 of Centruroides scorpion venom in a mouse neutralization assay.
The manufacturing procedures that contribute to the reduction of risk of viral transmission include pepsin digestion, ammonium sulfate precipitation/heat treatment and nanofiltration. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ANASCORP is composed of venom-specific F(ab')2 fragments of immunoglobulin G (IgG) that bind and neutralize venom toxins, facilitating redistribution away from target tissues and elimination from the body.1
12.3 Pharmacokinetics
Eight clinically healthy volunteers (6 males and 2 females, age: 17 to 26 years) received a bolus intravenous dose of 47.5 mg of Centruroides (Scorpion) Immune F(ab’)2, (Equine) Injection. Blood samples were collected till 504 hours (21 days) and pharmacokinetic parameters were estimated by non-compartmental analysis which are summarized in Table 2.
Table 2. Pharmacokinetic parameters of scorpion antivenom Parameters Mean ± sd
AUC(0-∞)(µghr/mL) 706 ± 352 Clearance (mL/hr) 83.5 ± 38.4 Half-life (hrs) 159 ± 57 Vss (liters) 13.6 ± 5.4 -
14 CLINICAL STUDIES
The efficacy of ANASCORP was assessed in a prospective double-blind randomized placebo-controlled study, four open-label studies and one retrospective study in various treatment settings in the United States and Mexico, where scorpion envenomation is common. A total of 1534 patients ranging from less than one month to 90 years old were treated. The majority of patients (78%, 1204/1534) were pediatric, ranging from less than one month to 18.7 years of age. Male (52.3%) and female patients (47.7%) were equally represented. Treatment success was determined by resolution of clinically important signs of scorpion envenomation within four hours of starting infusion. The randomized placebo study enrolled 15 subjects, eight to the ANASCORP treated group and seven to the placebo. The symptom resolution success rate was 100% for the ANASCORP treated and 14.3% for the placebo group.
A retrospective hospital chart review provided historical data from envenomated patients (N=97) who did not receive antivenom but were treated with sedatives and supportive care for symptoms of envenomation. These data were used as a historical control for expected outcomes in the absence of antivenom treatment. The historical controls were pediatric patients admitted to two pediatric intensive care units between 1990 and 2003 for the treatment of scorpion envenomation with supportive care only. The proportion of patients that required intensive care support four hours after intensive care unit admission, and the overall duration of the intensive care support requirement were calculated.
Overall, 95-100% of patients were relieved of systemic signs associated with scorpion envenomation in less than four hours after initiating ANASCORP treatment. In the historical control database, only 3.1% of patients experienced relief of symptoms within 4 hours of hospital admission.
In 1396/1534 patients the mean time from start of ANASCORP infusion to resolution of clinical signs and symptoms of envenomation was 1.42 hours (0.2 to 20.5 hours). Pediatric patients generally experienced a slightly faster time to resolution (1.28 ± 0.8 hours) compared with that of adult patients (1.91 ±1.4 hours). The time to resolution of symptoms was not affected by use of sedatives (474 patients who received sedatives resolved in 1.49 ± 1.1 hours and 922 patients who did not receive sedatives resolved in 1.38 ± 0.9 hours). -
15 REFERENCES
1. Krifi M.H, Savin S, Debray M, Bon C, Ayeb M.E, Choumet V. Pharmacokinetic studies of scorpion venom before and after antivenom immunotherapy. Toxicon, 2005; 45: 187–198.
2. Curry SC, Vance MV, Ryan PJ, Kunkel DB, Northey WT. Envenomation by the scorpion Centruroides sculpturatus, J Toxicol Clin Toxicol , 1983-1984; 21(4-5): 417-49.
3. Gibly R, Williams M, Walter FG, McNally J, Conroy C, Berg RA. Continuous intravenous midazolam infusion for Centruroides exilicauda scorpion envenomation. Ann Emerg Med,1999;34(5):620-5.
4. Vasquez H, Chavez-Haro A, Garcia-Ubbelohde W, et al., Pharmacokinetics of a F(ab’)2 scorpion antivenin in healthy human volunteers, Toxicon, 2005;46: 797-805.
5. Boyer LV, Theodorou AA, Berg RA, Mallie J. Antivenom for Critically Ill Children with Neurotoxicity from Scorpion Stings. N Engl J Med,2009;360:2090-8.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ANASCORP is supplied as a sterile lyophilized preparation in a single-use vial. When reconstituted, each vial contains not more than 3.8 milligrams per milliliter of protein, and not less than 150 mouse LD50 neutralizing units.
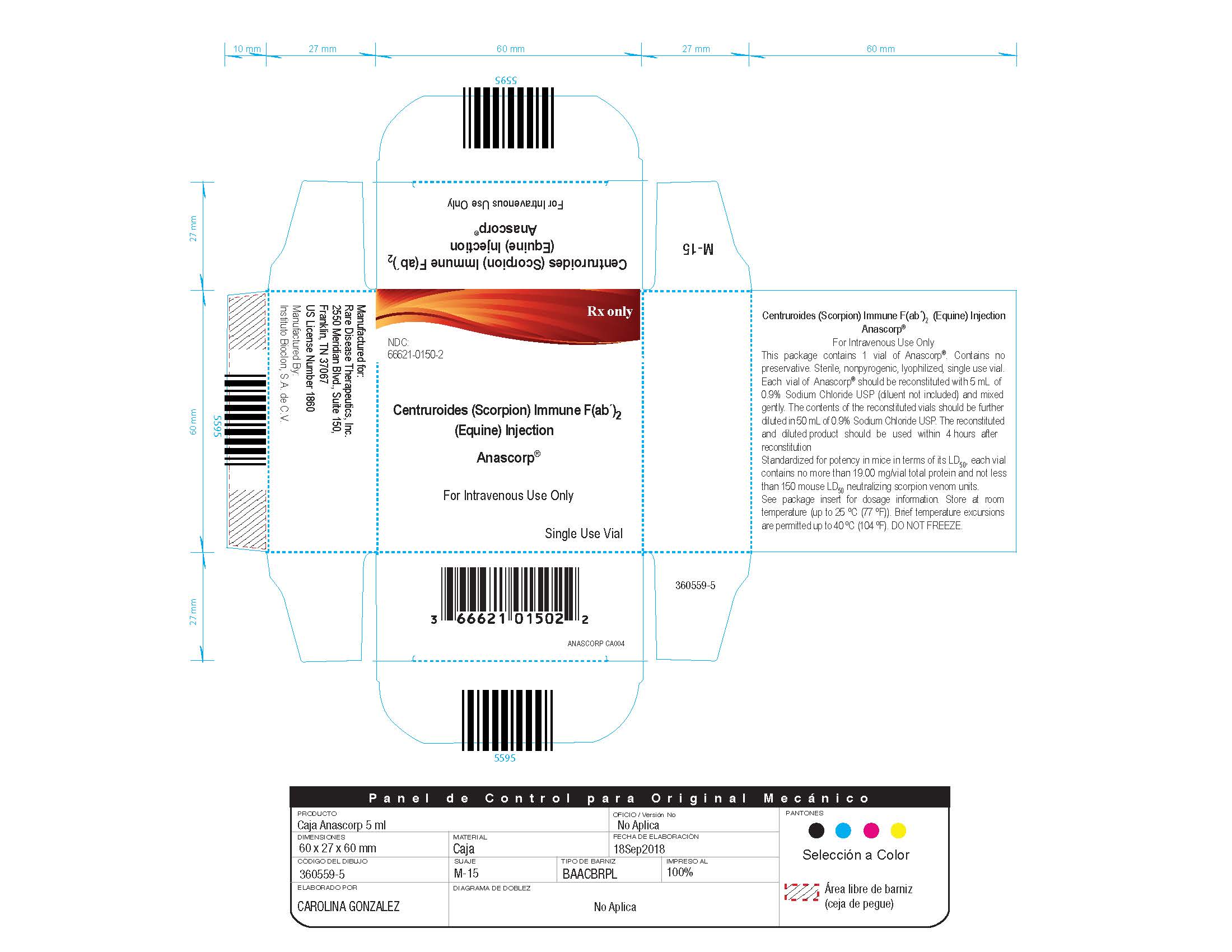
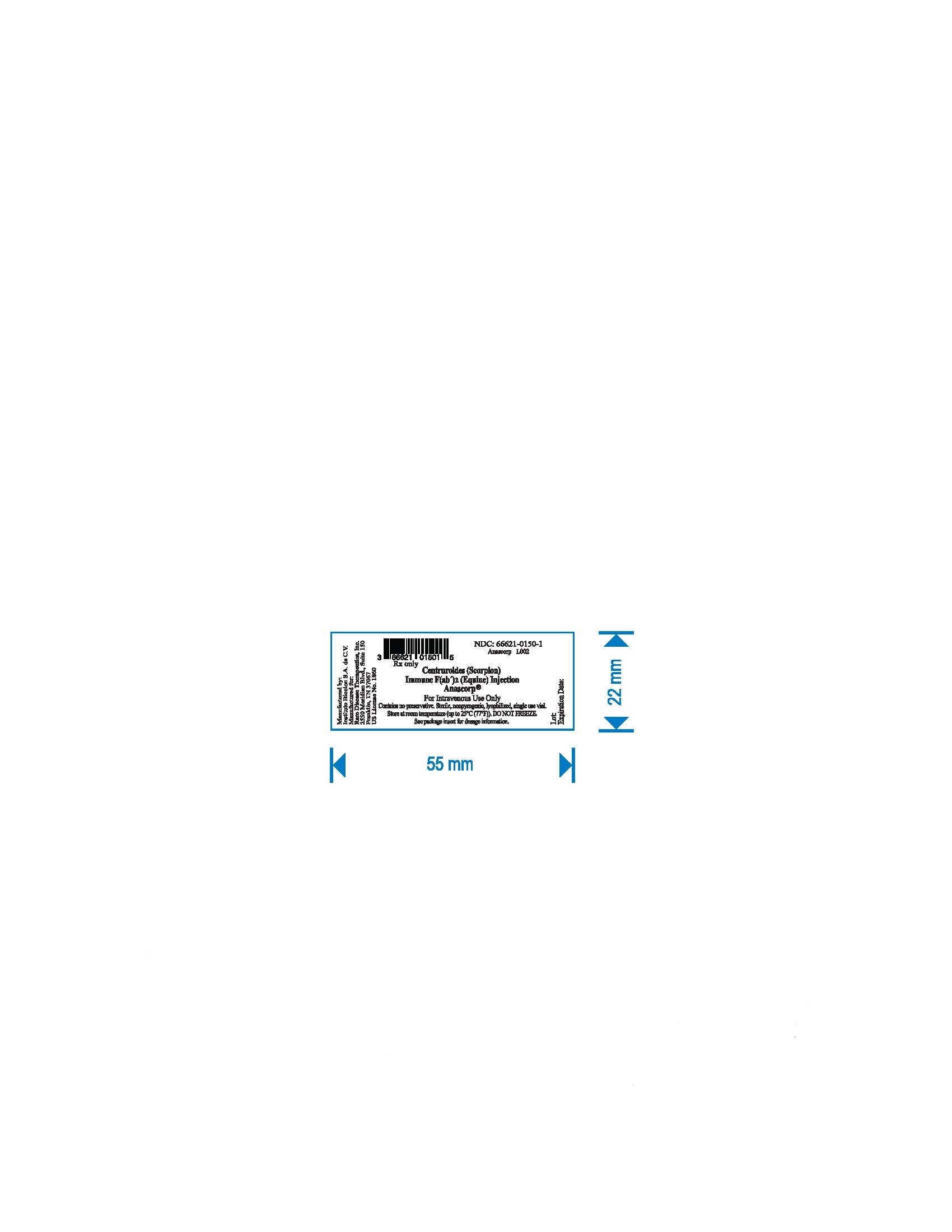
Each carton NDC: 66621-0150-2 contains 1 vial of ANASCORP NDC: 66621-0150-1.
- Store at room temperature (up to 25 ºC (77 ºF)). Brief temperature excursions are permitted up to 40 ºC (104ºF).
- DO NOT FREEZE.
- Discard partially used vials.
-
17 PATIENT COUNSELING INFORMATION
Advise patients to contact the physician or emergency department immediately if they experience any signs and symptoms of delayed allergic reactions or serum sickness up to 14 days following hospital discharge. Symptoms include rash, pruritus, joint pain, arthralgia, fever, lymphoadenopathy, and malaise.
Manufactured by:
Instituto Bioclon S.A. de C.V.
Tlalpan CDMX, Mexico
Manufactured for:
Rare Disease Therapeutics, Inc.
2550 Meridian Blvd., Suite 150
Franklin, TN 37067
www.raretx.com
U.S. License No. 1860
RDT Part No. ANASCORP PI004
Silanes Part No. 360557-5
PACKAGE LABEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANASCORP
centruroides (scorpion) immune f(ab)2 (equine) injection, powder, lyophilized, for solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 66621-0150 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CENTRUROIDES FAB2 ANTIVENIN (EQUINE) (UNII: DDA050FCEA) (CENTRUROIDES FAB2 ANTIVENIN (EQUINE) - UNII:DDA050FCEA) CENTRUROIDES FAB2 ANTIVENIN (EQUINE) 3.8 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66621-0150-2 1 in 1 CARTON 1 NDC: 66621-0150-1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125335 08/03/2011 Labeler - Rare Disease Therapeutics, Inc (966133100) Registrant - Rare Disease Therapeutics, Inc (966133100) Establishment Name Address ID/FEI Business Operations Instituto Bioclon S.A. de C.V. 811974559 manufacture(66621-0150)
Trademark Results [ANASCORP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ANASCORP 78825607 3403334 Live/Registered |
LABORATORIOS SILANES, S.A. DE C.V. 2006-02-28 |
 ANASCORP 78295395 not registered Dead/Abandoned |
Pharmasil, Inc. 2003-09-03 |
 ANASCORP 76213985 not registered Dead/Abandoned |
Orphan Pharmaceuticals, U.S.A., Inc. 2001-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.