CEFTRIAXONE- ceftriaxone sodium injection, powder, for solution
Ceftriaxone by
Drug Labeling and Warnings
Ceftriaxone by is a Prescription medication manufactured, distributed, or labeled by Samson Medical Technologies, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CEFTRIAXONE FOR INJECTION safely and effectively. See full prescribing information for CEFTRIAXONE FOR INJECTION.
CEFTRIAXONE FOR INJECTION, USP
Ceftriaxone for Injection, for intravenous use
Initial U.S. Approval: 1984PHARMACY BULK PACKAGE -
NOT FOR DIRECT INFUSIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of ceftriaxone and other antibacterial drugs, ceftriaxone should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (12.4)
INDICATIONS AND USAGE
Ceftriaxone for Injection, USP is a cephalosporin antibacterial indicated for the treatment of the following infections caused by susceptible isolates of the designated bacteria: Lower Respiratory Tract Infections (1.1); Skin and Skin Structure Infections (1.2); Complicated and Uncomplicated Urinary Tract Infections (1.3); Pelvic Inflammatory Disease (1.4); Bacterial Septicemia (1.5); Bone and Joint infections (1.6); Intra-abdominal Infections (1.7); Meningitis (1.8); and Surgical Prophylaxis (1.9).
DOSAGE AND ADMINISTRATION
For intravenous use only over approximately 30 minutes. (2)
THIS IS A PHARMACY BULK PACKAGE – NOT FOR DIRECT INJECTION.
Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof. Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of ceftriaxone (2.1).
- * Patients with hepatic impairment and significant renal impairment should not receive more than 2 grams per day of ceftriaxone.
Recommended Dosing Schedule for Ceftriaxone for Injection, USP Site and Type of
InfectionDose Frequency Total Daily Dose Usual Adult Dose 1 to 2 grams Once a day or in
equally divided doses
every 12 hoursShould not
exceed 4 grams*Surgical prophylaxis 1 gram I.V.
once½ to 2 hours before
surgeryMeningitis 100 mg per kg Once a day or in
equally divided doses
every 12 hoursShould not
exceed 4 grams*Skin and Skin Structure
Infections50 mg per kg to
75 mg per kgOnce a day or in
equally divided doses
every 12 hoursShould not
exceed 2 gramsSerious Infections other
than Meningitis50 mg per kg to
75 mg per kgEvery 12 hours Should not
exceed 2 gramsCeftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof. Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of ceftriaxone. (2.1)
DOSAGE FORMS AND STRENGTHS
- Pharmacy Bulk Package bags, 100 grams (3)
THIS IS A PHARMACY BULK PACKAGE – NOT FOR DIRECT INJECTION
CONTRAINDICATIONS
- Anaphylaxis to ceftriaxone or other cephalosporin class antibacterials, penicillins or other beta-lactam antibacterials (4.1)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Include anaphylaxis and serious skin reactions. Cross hypersensitivity may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue the drug (5.1).
- Interaction with Calcium-containing products: Precipitation can occur. Do not administer simultaneously with calcium-containing I.V. solutions (5.2).
- Clostridium difficile-associated diarrhea: May range from mild diarrhea to fatal colitis. Evaluate if diarrhea occurs (5.3).
- Hemolytic Anemia: Severe cases of hemolytic anemia, including fatalities in adults and children, have been reported. If anemia is diagnosed, discontinue the drug until the etiology is determined. (5.4)
ADVERSE REACTIONS
The most common adverse reactions occurring in >2% of patients receiving ceftriaxone include diarrhea, eosinophilia, thrombocytosis, leukopenia, and elevations of SGOT and SGPT. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Samson Medical Technologies, L.L.C. at 1-877-418-3600 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Hepatic impairment: Patients with both hepatic and renal impairment should not receive more than 2 grams of ceftriaxone per day. (5.7)
- Renal Impairment: This formulation of Ceftriaxone for Injection USP – Pharmacy Bulk Package bag, SmartPak® should not be used in renally impaired patients who require less than the 1 gram dose of ceftriaxone. Patients with both hepatic and renal impairment should not receive more than 2 grams of ceftriaxone per day. (5.7)
- Pediatric Patients: This formulation of Ceftriaxone for Injection USP – Pharmacy Bulk Package bag, SmartPak® should not be used in pediatric patients who require less than the 1 gram adult dose of ceftriaxone. (2.2, 8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Lower Respiratory Tract Infections
1.2 Skin and Skin Structure Infections
1.3 Complicated and Uncomplicated Urinary Tract Infections
1.4 Pelvic Inflammatory Disease
1.5 Bacterial Septicemia
1.6 Bone and Joint Infections
1.7 Intra-abdominal Infections
1.8 Meningitis
1.9 Surgical Prophylaxis
2 DOSAGE AND ADMINISTRATION
2.1 Adult Population
2.2 Pediatric Patients
2.3 Preparation for Use of Ceftriaxone for Injection, USP, Pharmacy Bulk Package bags, SmartPak®
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Anaphylaxis to Ceftriaxone or the Cephalosporin Class of Antibacterials, Penicillins or Other Beta-Lactam Antibacterials
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions to Ceftriaxone, Cephalosporins, Penicillins or Other Drugs
5.2 Interaction with Calcium-Containing Products
5.3 Clostridium difficile-associated Diarrhea
5.4 Hemolytic Anemia
5.5 Gallbladder Sonogram Abnormalities
5.6 Patients with Hepatic and Renal Impairment
5.7 Pancreatitis
5.8 Development of Drug-Resistant Bacteria
5.9 Alterations in Prothrombin Time
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
6.3 Cephalosporin-class Adverse Reactions
7 DRUG INTERACTIONS
7.1 Vancomycin, Amsacrine, Aminoglycosides, and Fluconazole
7.2 Calcium-containing Products
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Animal Pharmacology
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ceftriaxone for Injection and other antibacterial drugs, Ceftriaxone for Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ceftriaxone for Injection is indicated for the treatment of the following infections when caused by susceptible bacteria:
1.1 Lower Respiratory Tract Infections
Lower respiratory tract infections caused by Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Escherichia coli, Enterobacter aerogenes, Proteus mirabilis or Serratia marcescens.
1.2 Skin and Skin Structure Infections
Skin and skin structure infections caused by Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, Viridans group streptococci, Escherichia coli, Enterobacter cloacae, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii1, Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter calcoaceticus, Bacteroides fragilis1 or Peptostreptococcus species.
- 1 The efficacy for these organisms in this organ system were studied in fewer than ten infections.
1.3 Complicated and Uncomplicated Urinary Tract Infections
Complicated and uncomplicated urinary tract infections caused by Escherichia coli, Proteus mirabilis, Proteus vulgaris, Morganella morganii or Klebsiella pneumoniae.
1.4 Pelvic Inflammatory Disease
Pelvic inflammatory disease caused by Neisseria gonorrhoeae. Ceftriaxone sodium, like other cephalosporins, has no activity against Chlamydia trachomatis. Therefore, when cephalosporins are used in the treatment of patients with pelvic inflammatory disease and Chlamydia trachomatis is one of the suspected pathogens, appropriate antichlamydial coverage should be added.
1.5 Bacterial Septicemia
Bacterial septicemia caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae or Klebsiella pneumoniae.
1.6 Bone and Joint Infections
Bone and joint infections caused by Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae or Enterobacter species.
1.7 Intra-abdominal Infections
Intra-abdominal infections caused by Escherichia coli, Klebsiella pneumoniae, Bacteroides fragilis, Clostridium species or Peptostreptococcus species.
1.8 Meningitis
Meningitis caused by Haemophilus influenzae, Neisseria meningitidis or Streptococcus pneumoniae. Ceftriaxone sodium has also been used successfully in a limited number of cases of meningitis and shunt infection caused by Staphylococcus epidermidis and Escherichia coli; however, the efficacy for these organisms in this organ system were studied in fewer than ten infections.
1.9 Surgical Prophylaxis
The preoperative administration of a single 1 gram dose of ceftriaxone may reduce the incidence of postoperative infections in patients undergoing surgical procedures classified as contaminated or potentially contaminated (e.g., vaginal or abdominal hysterectomy or cholecystectomy for chronic calculous cholecystitis in high-risk patients, such as those over 70 years of age, with acute cholecystitis not requiring therapeutic antimicrobials, obstructive jaundice or common duct bile stones) and in surgical patients for whom infection at the operative site would present serious risk (e.g., during coronary artery bypass surgery). Although ceftriaxone sodium has been shown to have been as effective as cefazolin in the prevention of infection following coronary artery bypass surgery, no placebo-controlled trials have been conducted to evaluate any cephalosporin antibacterial in the prevention of infection following coronary artery bypass surgery.
-
2 DOSAGE AND ADMINISTRATION
Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof.
Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of Ceftriaxone.
THIS PHARMACY BULK PACKAGE REQUIRES RECONSTITUTION WITH STERILE WATER FOR INJECTION, USP TO A CONCENTRATION OF 100 mg per mL AND FURTHER DILUTION IN 50 mL OF A COMPATIBLE SOLUTION.
THIS IS A PHARMACY BULK PACKAGE – NOT FOR DIRECT INJECTION USE THIS FORMULATION OF CEFTRIAXONE ONLY IN PATIENTS WHO REQUIRE A 1 GRAM DOSE.
2.1 Adult Population
Ceftriaxone for Injection, USP, Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof.
Ceftriaxone for Injection USP Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of Ceftriaxone.
Ceftriaxone for Injection should be reconstituted with Sterile Water for Injection, USP to a concentration of 100 mg per mL and further diluted in 50 mL of a compatible solution.
The recommended adult dosages are outlined in Table 1. Ceftriaxone for Injection, USP, Pharmacy Bulk Package SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof.
Ceftriaxone should be administered intravenously (I.V.) over approximately 30 minutes.
The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required. When treating infections caused by Streptococcus pyogenes, therapy should be continued for at least 10 days.
Table 1: Recommended Dosing Schedule for Ceftriaxone for Injection, USP Site and Type of
InfectionDose Frequency Total Daily Dose Usual Adult Dose 1 gram to 2 grams Once a day or in equally divided doses every 12 hours Should not exceed 4 grams* Surgical prophylaxis 1 gram I.V. once ½ to 2 hours before surgery Skin and Skin Structure Infections 50 to 75 mg per kg Once a day or in equally divided doses every 12 hours Should not exceed 2 grams Meningitis 100 mg per kg Once a day or in equally divided doses every 12 hours Should not exceed 4 grams* Serious Infections other than Meningitis 50 to 75 mg per kg Every 12 hours Should not exceed 2 grams *Patients with hepatic impairment and significant renal impairment should not receive more than 2 grams per day of ceftriaxone.
**This formulation of Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of ceftriaxone.
2.2 Pediatric Patients
To prevent unintentional overdose, this product should not be used in pediatric patients who require less than the adult 1 gram dose of ceftriaxone. [see Use in Specific Populations (8.4)]
2.3 Preparation for Use of Ceftriaxone for Injection, USP, Pharmacy Bulk Package bags, SmartPak®
Ceftriaxone for Injection, USP Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof.
Ceftriaxone for Injection USP, Pharmacy Bulk Package SmartPak® bag should not be used in patients who require less than the 1 gram dose of ceftriaxone.
Directions for Proper Use of a Pharmacy Bulk Package
- NOT FOR DIRECT INFUSION. The Pharmacy Bulk Package is for use in the hospital pharmacy admixture service only in a suitable work area, such as a laminar flow hood. Using aseptic technique, the container closure may be penetrated only one time after reconstitution using a suitable sterile dispensing set or transfer device that allows measured dispensing of the contents. Use of a syringe and needle is not recommended as it may cause leakage. The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of 4 HOURS from initial reconstitution port closure entry is permitted to complete fluid transfer operations. This time limit should begin with the introduction of the solvent or diluent into the Pharmacy Bulk Package. Discard any unused portion after 4 HOURS. This pharmacy bulk package is not intended to be dispensed as a unit
- PRIOR TO RECONSTITUTION:DO NOT USE THE INNER BAG IF PARTICULATE OR FOREIGN MATTER IS PRESENT, IF THE DRY POWDER IS DARK YELLOW OR BROWN, IF THE SEALS ARE NOT INTACT, OR IF THERE IS ANY OTHER DAMAGE TO THE BAG. IN SUCH CASES, DISCARD THE BAG IMMEDIATELY.
- After initial reconstitution port entry, use entire contents of the Pharmacy Bulk Package promptly. Any unused portion must be discarded after 4 HOURS.
- Gather the following items prior to the reconstitution of the product: Appropriate number of bags of Sterile Water for Injection and, depending upon the method of filling, appropriate sterile tubing and adapters.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Use only if solution is clear.
- Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute Ceftriaxone for Injection SmartPak® Pharmacy Bulk Package or to further dilute a reconstituted solution for intravenous administration. Particulate formation can result.
INSTRUCTION FOR RECONSTITUTION OF THE PHARMACY BULK PACKAGE BAG SmartPak®
The entire contents of the bag and the preparation process (reconstitution and dilution) should be completed within 4 hours of initial entry.
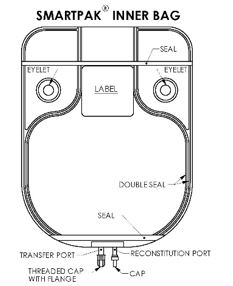
- Document the date and time reconstitution starts in the designated place on the container label. The entire contents of the bag must be used within 4 hours from the time of initial entry.
- Remove the translucent unthreaded cap from the reconstitution (smaller) port and discard it.
- Reconstitute the powder through the reconstitution (smaller) port, using Sterile Water for Injection according to the Table 2 below:
- * Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute or further dilute reconstituted ceftriaxone because a precipitate can form. STERILE WATER FOR INJECTION IS THE ONLY RECOMMENDED DILUENT FOR RECONSTITUTION. [see Warnings and Precautions [(5.2)].
Table 2: Reconstitution Table SmartPak® Bag
SizeAmount of Sterile
Water*Approximate Concentration 100 grams 950 mL 100 mg/mL (1 g/10 mL) - After reconstitution is complete, remove the transfer needle from the reconstitution port.
- Place the bag on a flat surface of a laminar flow hood and mix for at least 15 minutes by rocking gently from side to side. CAUTION: To avoid possible leakage caused by the heavy weight of the added water, do not shake vigorously or pull strongly on the bag.
- When foam dissipates, visually inspect the bag to verify the solution is clear, colorless to pale yellow and free of particulate matter. DO NOT USE THE INNER BAG IF PARTICULATE OR FOREIGN MATTER IS PRESENT.
- Unscrew the clear threaded cap from the transfer (larger) port and discard it. Attach sterile tubing and filling adapter unit to the transfer port.
- Reconstituted solution can now be transferred using the transfer port and the filling adapter.
It should be noted that the spike placed into the transfer port of the Pharmacy Bulk Package SmartPak® is NEVER removed during this procedure and that the reconstitution port is self-sealing.
-
SPL UNCLASSIFIED SECTION
Dilution
- Hang the bag from two eyelets.
- Following reconstitution, transfer 10 mL of the reconstituted solution into transfusion bags, each containing 50 mL of one of the compatible solutions below.
Compatible solutions for dilution are the following:
Sodium Chloride Injection, USP
5% Dextrose Injection, USP - Dilution should be completed within the 4 hour preparation process.
- When diluted according to the instructions above, ceftriaxone is stable for 48 hours at room temperature or for 10 days if stored under refrigeration (5°C or 41°F).
-
SPL UNCLASSIFIED SECTION
Important Administration Instructions
- Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute Ceftriaxone for Injection SmartPak® Pharmacy Bulk Package or to further dilute a reconstituted solution for intravenous administration. Particulate formation can result. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same intravenous administration line. Ceftriaxone for Injection, USP must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions, such as parenteral nutrition via a Y-site. However, in patients other than neonates, Ceftriaxone for Injection, USP and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid [see Warnings and Precautions (5.2)].
- Prior to administration, parenteral products should be inspected visually for particulate matter and discoloration whenever solution and container permit.
- Administer diluted ceftriaxone solutions intravenously over approximately 30 minutes.
- After the indicated stability times, unused portions of solutions should be discarded.
- Vancomycin, amsacrine, aminoglycosides and fluconazole are physically incompatible with ceftriaxone in admixtures. When any of these drugs are to be administered concomitantly with ceftriaxone by intermittent intravenous infusion, it is recommended that they be given sequentially with thorough flushing of the intravenous lines with 0.9% Sodium Chloride Injection or 5% Dextrose in Water (D5W) between the administrations.
- Ceftriaxone for Injection should not be physically mixed with or piggybacked into solutions containing other antimicrobial drugs due to possible incompatibility [see Drug Interactions (7.1)].
- There have been no reports of an interaction between ceftriaxone and oral calcium-containing products or interaction between intramuscular ceftriaxone and calcium-containing products (intravenous or oral).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Anaphylaxis to Ceftriaxone or the Cephalosporin Class of Antibacterials, Penicillins or Other Beta-Lactam Antibacterials
Ceftriaxone for Injection, USP is contraindicated in patients who have a history of anaphylaxis to ceftriaxone or the cephalosporin class of antibacterials, penicillins, or other beta-lactam antibacterials [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions to Ceftriaxone, Cephalosporins, Penicillins or Other Drugs
Serious, occasionally fatal, hypersensitivity (anaphylactic) reactions have been reported with ceftriaxone. Before therapy with Ceftriaxone for Injection, USP is instituted, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to ceftriaxone, cephalosporins, penicillins, or other drugs. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibacterials has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to Ceftriaxone for Injection, USP occurs, discontinue the drug. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures including oxygen, corticosteroids, intravenous fluids, intravenous antihistamines, pressor amines, and airway management, as clinically indicated.
5.2 Interaction with Calcium-Containing Products
Precipitation of ceftriaxone-calcium can occur when Ceftriaxone for Injection, USP is mixed with calcium-containing solutions in the same intravenous administration line. Ceftriaxone for Injection must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions, such as parenteral nutrition via a Y-site. However, in patients other than neonates, Ceftriaxone for Injection, USP and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with 0.9% Sodium Chloride Injection or D5W. In vitro studies using adult and neonatal plasma from umbilical cord blood demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium. [see Drug Interactions (7.2)]
5.3 Clostridium difficile-associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ceftriaxone, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B, which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary, since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Hemolytic Anemia
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia while on Ceftriaxone for Injection, USP, the diagnosis of a cephalosporin-associated anemia should be considered and Ceftriaxone for Injection, USP stopped until the etiology is determined.
5.5 Gallbladder Sonogram Abnormalities
There have been reports of sonographic abnormalities in the gallbladder of patients treated with ceftriaxone sodium; some of these patients also had symptoms of gallbladder disease. These abnormalities appear on sonography as an echo without acoustical shadowing suggesting sludge or as an echo with acoustical shadowing, which may be misinterpreted as gallstones. The chemical nature of the sonographically detected material has been determined to be predominantly a ceftriaxone-calcium salt. The condition appears to be transient and reversible upon discontinuation of ceftriaxone sodium and institution of conservative management. Therefore, ceftriaxone should be discontinued in patients who develop signs and symptoms suggestive of gallbladder disease and/or the sonographic findings described above.
5.6 Patients with Hepatic and Renal Impairment
Hepatic Impairment: In patients with both hepatic and significant renal disease, Ceftriaxone for Injection dosage should not exceed 2 grams daily.
Renal Impairment: This formulation of Ceftriaxone for Injection USP – Pharmacy Bulk Package bags SmartPak® should not be used in renally impaired patients who require less than the 1 gram dose of ceftriaxone. In patients with both hepatic impairment and significant renal disease, Ceftriaxone for Injection dosage should not exceed 2 grams per day.
5.7 Pancreatitis
Cases of pancreatitis, possibly secondary to biliary obstruction, have been reported in patients treated with ceftriaxone sodium. Most patients presented with risk factors for biliary stasis and biliary sludge (preceding major therapy, severe illness, total parenteral nutrition). A cofactor role of ceftriaxone-related biliary precipitation cannot be ruled out.
5.8 Development of Drug-Resistant Bacteria
Prescribing Ceftriaxone for Injection, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. As with other antibacterial drugs, use of Ceftriaxone for Injection may result in overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
5.9 Alterations in Prothrombin Time
Alterations in prothrombin times have occurred in patients treated with ceftriaxone sodium. Patients with impaired vitamin K synthesis or low vitamin K stores (e.g., chronic hepatic disease and malnutrition) may require monitoring of prothrombin time during Ceftriaxone for Injection treatment. Vitamin K administration (10 mg weekly) may be necessary if the prothrombin time is prolonged before or during therapy.
-
6 ADVERSE REACTIONS
The following serious adverse reactions to ceftriaxone are described below and elsewhere in the labeling:
- Hypersensitivity reactions [see Warnings and Precautions (5.1)]
- Ceftriaxone-calcium precipitates [see Warnings and Precautions (5.2) and Drug Interactions (7.2)]
- Clostridium difficile-associated diarrhea [see Warnings and Precautions (5.3)]
- Hemolytic anemia [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The following reactions occurred in ≤ 6% of the patients:
- Local reactions – pain, induration, tenderness, and phlebitis after intravenous administration.
- Hypersensitivity – rash, pruritus, fever or chills.
- Hematologic – eosinophilia, thrombocytosis, leukopenia, anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia, and prolongation of the prothrombin time.
- Gastrointestinal – diarrhea, nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.3)].
- Hepatic – elevations of SGOT, SGPT, alkaline phosphatase, bilirubin.
- Renal – elevations of the BUN, creatinine, and the presence of casts in the urine.
- Central Nervous System – headache or dizziness.
- Genitourinary – moniliasis or vaginitis.
- Miscellaneous – diaphoresis and flushing.
- Ceftriaxone-calcium precipitates – Cases of fatal reactions with ceftriaxone-calcium precipitates in lung and kidneys in neonates have been described. In some cases the infusion lines and times of administration of ceftriaxone and calcium-containing solutions differed [see Warnings and Precautions (5.2)and Drug Interactions (7.2)].
- Other observed adverse reactions - abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
6.2 Postmarketing Experience
The following adverse reactions have been reported during postapproval use of ceftriaxone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to readily estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal – stomatitis and glossitis
- Genitourinary – oliguria
- Dermatologic – exanthema, allergic dermatitis, urticaria, edema
Cases of severe cutaneous adverse reactions (erythema multiforme, Stevens-Johnson syndrome or Lyell’s syndrome/toxic epidermal necrolysis) have been reported.
Anaphylaxis (including anaphylactic shock, transient leukopenia, neutropenia, agranulocytosis and thrombocytopenia) has been reported.
6.3 Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with ceftriaxone, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibacterials:
- Adverse Reactions: Allergic reactions, drug fever, serum sickness-like reaction, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, and superinfection.
- Altered Laboratory Tests: Positive direct Coombs’ test, false-positive test for urinary glucose, and elevated LDH.
-
7 DRUG INTERACTIONS
7.1 Vancomycin, Amsacrine, Aminoglycosides, and Fluconazole
Vancomycin, amsacrine, aminoglycosides, and fluconazole are physically incompatible with ceftriaxone in admixtures [see Dosage and Administration (2.3)].
7.2 Calcium-containing Products
Precipitation of ceftriaxone-calcium can occur when Ceftriaxone for Injection is mixed with calcium-containing solutions in the same intravenous administration line. Ceftriaxone for Injection, USP must not be administered simultaneously with calcium-containing intravenous solutions. Ceftriaxone for Injection and calcium-containing solutions can be administered sequentially [see Warnings and Precautions (5.2)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
Reproductive studies have been performed in mice, rats, and primates at intravenous doses of 625, 586, and 84 mg/kg/day, respectively without evidence of embryotoxicity, fetotoxicity, or teratogenicity. These doses are approximately 1.5, 2.8, and 0.8 times the recommended clinical dose of 2 grams/day based on body surface area comparisons.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Ceftriaxone was tested in a Segment III (pre-postnatal) study in rats at intravenous doses of up to 586 mg/kg/day approximately 2.8 times (mg/m2 comparison) the recommended daily dose of 2 grams/day. No adverse effects were noted on various reproductive parameters during gestation and lactation, including postnatal growth, functional behavior, and reproductive ability of the offspring.
8.3 Nursing Mothers
Ceftriaxone is excreted in human breast milk. Caution should be exercised when Ceftriaxone for Injection is administered to a nursing woman.
8.4 Pediatric Use
Ceftriaxone for Injection USP, Pharmacy Bulk Package bag, SmartPak® should not be used in pediatric patients who require less than the 1 gram dose of ceftriaxone. To prevent unintentional overdose, this product should not be used in pediatric patients who require less than the adult 1 gram dose of ceftriaxone.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of ceftriaxone sodium, 32% were 60 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Ceftriaxone is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
The pharmacokinetics of ceftriaxone were only minimally altered in geriatric patients compared to healthy adult subjects, and dosage adjustments are not necessary for geriatric patients with ceftriaxone dosages up to 2 grams per day [see Clinical Pharmacology (12)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Ceftriaxone for Injection, USP, Pharmacy Bulk Package bag SmartPak® should be used only in patients who require a 1 gram dose and not any fraction thereof.
Ceftriaxone for Injection USP, Pharmacy Bulk Package bag SmartPak® should not be used in patients who require less than the 1 gram dose of ceftriaxone.
Ceftriaxone for Injection, USP, is a sterile, semisynthetic, broad-spectrum cephalosporin antibacterial for intravenous administration. Ceftriaxone sodium is (6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-8-oxo-3-[[(1,2,5,6-tetrahydro-2-methyl-5,6-dioxo-as-triazin-3-yl)thio]methyl]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 72-(Z)-(O-methyloxime), disodium salt, sesquaterhydrate.
The chemical formula of ceftriaxone sodium is C18H16N8Na2O7S33.5H2O. It has a calculated molecular weight of 661.60 and the following structural formula:
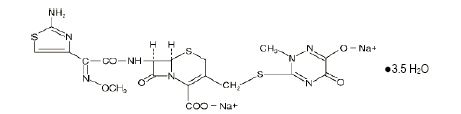
Ceftriaxone sodium is a white to yellowish-orange crystalline powder, which is readily soluble in water, sparingly soluble in methanol and very slightly soluble in ethanol. The pH of a 1% aqueous solution is approximately 6.7. The color of ceftriaxone sodium solutions ranges from light yellow to amber, depending on the length of storage and concentration. Each Pharmacy Bulk Package contains approximately 83 mg (3.6 mEq) of sodium per 1 gram of ceftriaxone activity. Ceftriaxone for Injection, USP is supplied in 100 grams SmartPak® Pharmacy Bulk Packages bags equivalent. Each SmartPak® Pharmacy Bulk Package bag contains ceftriaxone sodium, USP equivalent to 100 grams of ceftriaxone.
BEFORE ADMINISTRATION, THIS PHARMACY BULK PACKAGE REQUIRES RECONSTITUTION USING STERILE WATER FOR INJECTION, USP TO A CONCENTRATION OF 100 mg per mL AND FURTHER DILUTION IN 50 mL OF A COMPATIBLE SOLUTION AND INFUSED INTRAVENOUSLY OVER 30 MINUTES.
THIS PRODUCT IS NOT INTENDED TO BE USED IN PEDIATRIC PATIENTS AND RENALLY IMPAIRED PATIENTS WHO REQUIRE LESS THAN A 1 GRAM DOSE.
A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture service and are restricted to the preparation of admixtures for intravenous infusion.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Average plasma concentrations of ceftriaxone following a single 30-minute intravenous (I.V.) infusion of a 0.5, 1 or 2 g dose in healthy subjects are presented in Table 3. Multiple intravenous doses ranging from 0.5 to 2 g at 12- to 24-hour intervals resulted in 15% to 36% accumulation of ceftriaxone above single dose values.
TABLE 3: Ceftriaxone Plasma Concentrations After Single Dose Administration
- * I.V. doses were infused at a constant rate over 30 minutes.
Dose/Route Average Plasma Concentrations (mcg/mL) 0.5 hr 1 hr 2 hr 4 hr 6 hr 8 hr 12 hr 16 hr 24 hr 0.5 gram I.V.* 82 59 48 37 29 23 15 10 5 1 gram I.V.* 151 111 88 67 53 43 28 18 9 2 grams I.V.* 257 192 154 117 89 74 46 31 15 Over a 0.15 to 3 g dose range in healthy adult subjects, the mean elimination half-life ranged from 5.8 to 8.7 hours, plasma clearance ranged from 0.58 to 1.45 L/hour, and renal clearance ranged from 0.32 to 0.73 L/hour.
Distribution
Ceftriaxone is reversibly bound to human plasma proteins and the binding of ceftriaxone decreases with increasing concentration from a value of 95% at plasma concentrations less than 25 mcg/mL to 85% at plasma concentration of 300 mcg/mL. Over a 0.15 to 3 g dose range in healthy adult subjects, the apparent volume of distribution ranged from 5.8 to 13.5 L.
Ceftriaxone crosses the blood placenta barrier.
Ceftriaxone penetrates the inflamed meninges of infants and pediatric patients. The average values of maximum plasma concentration, cerebrospinal fluid (CSF) concentrations, elimination half-life, plasma clearance and volume of distribution after a 50 mg/kg intravenous dose and after a 75 mg/kg intravenous dose in pediatric patients suffering from bacterial meningitis are shown in Table 4.
TABLE 4: Average Pharmacokinetic Parameters of Ceftriaxone in Pediatric Patients with Meningitis
50 mg/kg I.V. 75 mg/kg I.V. Maximum Plasma Concentrations (mcg/mL) 216 275 Elimination Half-life (hr) 4.6 4.3 Plasma Clearance (mL/hr/kg) 49 60 Volume of Distribution (mL/kg) 338 373 CSF Concentration – inflamed meninges (mcg/mL) 5.6 6.4 Range (mcg/mL) 1.3-18.5 1.3-44 Time after dose (hr) 3.7 (±1.6) 3.3 (±1.4) After a 1 gram intravenous dose, average concentrations of ceftriaxone, determined from 1 to 3 hours after dosing, were 581 mcg/mL in the gallbladder bile, 788 mcg/mL in the common duct bile, 898 mcg/mL in the cystic duct bile, 78.2 mcg/gram in the gallbladder wall compared to a corresponding concentration of 62.1 mcg/mL in plasma.
Excretion
Ceftriaxone concentrations in urine are shown in Table 5.
TABLE 5: Urinary Concentrations of Ceftriaxone After Single Dose Administration
Average Urinary Concentrations (mcg/mL) Dose/Route 0-2 hr 2-4 hr 4-8 hr 8-12 hr 12-24 hr 24-48 hr 0.5 gram I.V. 526 366 142 87 70 15 1 gram I.V. 995 855 293 147 132 32 2 grams I.V. 2692 1976 757 274 198 40 Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug, and the remainder was secreted in the bile and ultimately found in the feces as microbiologically inactive compounds.
The elimination of ceftriaxone is not altered by probenecid.
Special Populations
Average pharmacokinetic parameters of ceftriaxone in healthy subjects, elderly subjects, subjects with renal impairment, and subjects with liver disease are summarized in Table 6. Compared to healthy adult subjects, the pharmacokinetics of ceftriaxone were only minimally altered in elderly subjects and in patients with renal or hepatic impairment; therefore, dosage adjustments are not necessary for these patients with ceftriaxone dosages up to 2 grams per day. Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis. In 6 of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced, suggesting that plasma concentrations of ceftriaxone should be monitored in these patients to determine if dosage adjustments are necessary [see Dosage and Administration (2.1) and Warnings and Precautions (5.6)].
TABLE 6: Average Pharmacokinetic Parameters of Ceftriaxone in Humans
- * Dose ranged from 0.15 to 3 grams
- † Creatinine clearance.
Subject Group Elimination
Half-Life (hr)Plasma
Clearance (L/hr)Volume of Distribution
(L)Healthy Subjects* 5.8-8.7 0.58-1.45 5.8-13.5 Elderly Subjects (mean age, 70.5 yr) 8.9 0.83 10.7 Patients with Renal Impairment Hemodialysis Patients (0-5 mL/min)† 14.7 0.65 13.7 Severe (5-15 mL/min) 15.7 0.56 12.5 Moderate (16-30 mL/min) 11.4 0.72 11.8 Mild (31-60 mL/min) 12.4 0.70 13.3 Patients with Liver Disease 8.8 1.1 13.6 Drug Interactions
Interaction with Calcium: Two in vitro studies, one using adult plasma and the other neonatal plasma from umbilical cord blood, have been carried out to assess interaction of ceftriaxone and calcium. Ceftriaxone concentrations up to 1 mM (in excess of concentrations achieved in vivo following administration of 2 grams ceftriaxone infused over 30 minutes) were used in combination with calcium concentrations up to 12 mM (48 mg/dL). Recovery of ceftriaxone from plasma was reduced with calcium concentrations of 6 mM (24 mg/dL) or higher in adult plasma or 4 mM (16 mg/dL) or higher in neonatal plasma. This may be reflective of ceftriaxone-calcium precipitation.
12.4 Microbiology
Mechanism of Action
Ceftriaxone is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Ceftriaxone has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Mechanism of Resistance
Resistance to ceftriaxone is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability.
Interaction with Other Antimicrobials
In an in vitro study antagonistic effects have been observed with the combination of chloramphenicol and ceftriaxone.
Antibacterial Activity
Ceftriaxone has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections described in the INDICATIONS AND USAGE (1) section.
-
Gram-negative Bacteria
- Acinetobacter calcoaceticus
- Enterobacter aerogenes
- Enterobacter cloacae
- Escherichia coli
- Haemophilus influenzae
- Haemophilus parainfluenzae
- Klebsiella oxytoca
- Klebsiella pneumoniae
- Moraxella catarrhalis
- Morganella morganii
- Neisseria gonorrhoeae
- Neisseria meningitidis
- Proteus mirabilis
- Proteus vulgaris
- Pseudomonas aeruginosa
- Serratia marcescens
-
Gram-positive Bacteria
- Staphylococcus aureus
- Staphylococcus epidermidis
- Streptococcus pneumoniae
- Streptococcus pyogenes
- Viridans group streptococci
-
Anaerobic Bacteria
- Bacteroides fragilis
- Clostridium species
- Peptostreptococcus species
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for ceftriaxone. However, the efficacy of ceftriaxone in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
-
Gram-negative Bacteria
- Citrobacter diversus
- Citrobacter freundii
- Providencia species (including Providencia rettgeri)
- Salmonella species (including Salmonella typhi)
- Shigella species
-
Gram-positive Bacteria
- Streptococcus agalactiae
-
Anaerobic Bacteria
- Porphyromonas (Bacteroides) melaninogenicus
- Prevotella (Bacteroides) bivius
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method. 1,3 . The MIC values should be interpreted according to the criteria provided in Table 7.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. 2,3 This procedure uses paper disks impregnated with 30 mcg ceftriaxone to test the susceptibility of microorganisms to ceftriaxone. The disk diffusion interpretive criteria are provided in Table 7.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to ceftriaxone as MICs can be determined by a standardized agar test method.3,4 The MIC values obtained should be interpreted according to the criteria provided in Table 7.
Table 7: Susceptibility Test Interpretive Criteria for Ceftriaxone
- * Susceptibility interpretive criteria for Enterobacteriaceae are based on a dose of 1 gram IV q 24h. For isolates with intermediate susceptibility, use a dose of 2 grams IV q 24h in patients with normal renal function.
- † For Haemophilus influenzae, susceptibility interpretive criteria are based on a dose of 2 grams IV every 24 hours in patients with normal renal function.
- ‡ The current absence of data on resistant isolates precludes defining any category other than ‘Susceptible’. If isolates yield MIC results other than susceptible, they should be submitted to a reference laboratory for additional testing.
- § Disc diffusion interpretive criteria for ceftriaxone discs against Streptococcus pneumoniae are not available, however, isolates of pneumococci with oxacillin zone diameters of ≥20 mm are susceptible (MIC ≤ 0.06 mcg/mL) to penicillin and can be considered susceptible to ceftriaxone. Streptococcus pneumoniae isolates should not be reported as penicillin (ceftriaxone) resistant or intermediate based solely on an oxacillin zone diameter of ≤ 19 mm. The ceftriaxone MIC should be determined for those isolates with oxacillin zone diameters of ≤ 19 mm.
Pathogen Minimum Inhibitory Concentrations
(mcg/mL)Disk Diffusion Zone Diameters
(mm)S
Susceptible
I
Intermediate
R
Resistant
S
Susceptible
I
Intermediate
R
Resistant
Enterobacteriaceae* ≤ 1 2 ≥ 4 ≥ 23 20-22 ≤ 19 Haemophilus influenzae†‡ ≤ 2 - - ≥ 26 - - Neisseria gonorrhoeae‡ ≤ 0.25 - - ≥ 35 - - Neisseria meningitidis‡ ≤ 0.12 - - ≥ 34 - - Streptococcus pneumoniae§ meningitis isolates ≤ 0.5 1 ≥ 2 - - - Streptococcus pneumoniae§ non-meningitis isolates ≤ 1 2 ≥ 4 - - - Streptococcus species beta-hemolytic group‡ ≤ 0.5 - - ≥ 24 - - Viridans group streptococci ≤ 1 2 ≥ 4 ≥ 27 25-26 ≤ 24 Anaerobic bacteria (agar method) ≤ 1 2 ≥ 4 - - - Susceptibility of staphylococci to ceftriaxone may be deduced from testing only penicillin and either cefoxitin or oxacillin. A report of Susceptible indicates that the antimicrobial drug is likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration at the site of infection. A report of Intermediate indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial drug is not likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the test 1,2,3,4. Standard ceftriaxone powder should provide the following range of MIC values noted in Table 8. For the diffusion technique using the 30 mcg disk, the criteria in Table 8 should be achieved.
Table 8: Acceptable Quality Control Ranges for Ceftriaxone
QC Strain Minimum Inhibitory Concentrations
(mcg/mL)Disk Diffusion Zone Diameters
(mm)Escherichia coli ATCC 25922 0.03 – 0.12 29 – 35 Staphylococcus aureus ATCC 25923 - 22 – 28 Staphylococcus aureus ATCC 29213 1 – 8 - Haemophilus influenzae ATCC 49247 0.06 – 0.25 31 – 39 Neisseria gonorrhoeae ATCC 49226 0.004 – 0.015 39 – 51 Pseudomonas aeruginosa ATCC 27853 8 – 64 17 – 23 Streptococcus pneumoniae ATCC 49619 0.03 – 0.12 30 – 35 Bacteroides fragilis ATCC 25285 (agar method) 32 – 128 - Bacteroides thetaiotaomicron ATCC 29741 (agar method) 64 – 256 - -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Considering the maximum duration of treatment and the class of the compound, carcinogenicity studies with ceftriaxone in animals have not been performed. The maximum duration of animal toxicity studies was 6 months.
13.2 Animal Toxicology and/or Animal Pharmacology
Concretions consisting of the precipitated calcium salt of ceftriaxone have been found in the gallbladder bile of dogs and baboons treated with ceftriaxone. These appeared as a gritty sediment in dogs that received 100 mg/kg/day for 4 weeks.
A similar phenomenon has been observed in baboons but only after a protracted dosing period (6 months) at higher dose levels (335 mg/kg/day or more).
-
15 REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Tenth Edition. CLSI document M07-A10, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth Informational Supplement. CLSI document M100-S25, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Twelfth Edition. CLSI document M02-A12, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard – Eighth Edition. CLSI document M11-A8, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, PA 19087, USA, 2012.
- Barnett ED, Teele DW, Klein JO, et al. Comparison of Ceftriaxone and Trimethoprim-Sulfamethoxazole for Acute Otitis Media. Pediatrics. Vol. 99, No. 1, January 1997.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ceftriaxone for Injection, USP is available in the following SmartPak® Pharmacy Bulk Package bags:
100 grams* One Pharmacy Bulk Package bag Product No. 6100 NDC: 66288-6100-1.
*Each 100 gram Pharmacy Bulk Package bag contains sterile ceftriaxone sodium equivalent to 100 grams of ceftriaxone
SmartPak® system components are not made with natural rubber latex.
Precautions
As with other cephalosporins, reconstituted Ceftriaxone for Injection tends to darken depending on storage conditions within the stated recommendations. However, product potency is not adversely affected.
Use only if prepared solution is clear and free from particulate matter.
Storage Conditions
Prior to reconstitution, store Ceftriaxone for Injection sterile powder at room temperature 20º-25ºC (68º-77ºF) [see USP Controlled Room Temperature] and protected from light.
-
17 PATIENT COUNSELING INFORMATION
Patients should be advised that allergic reactions, including serious allergic reactions could occur and that serious reactions require immediate treatment and discontinuation of ceftriaxone. Patients should report to their health care provider any previous allergic reactions to ceftriaxone, cephalosporins, penicillins, or other similar antibacterials.
Advise patients of neurological adverse reactions that could occur with Ceftriaxone for Injection, USP use. Instruct patients to inform a healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, and seizures, for immediate treatment, dosage adjustment, or discontinuation of Ceftriaxone for Injection, USP.
Patients should be advised that diarrhea is a common problem caused by antibacterials, which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterials, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial. If this occurs, patients should contact a physician as soon as possible.
Patients should be counseled that antibacterial drugs including ceftriaxone, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Ceftriaxone for Injection, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ceftriaxone or other antibacterial drugs in the future.
Rx only
SmartPak is a registered trademark of Samson Medical Technologies, L.L.C.
C6100c
Manufactured for
SAMSON
MEDICAL TECHNOLOGIES L.L.C.
Cherry Hill, NJ 08003, USA
by
ACS Dobfar S.p.A.
20067 Tribiano (Milano) Italy -
PRINCIPAL DISPLAY PANEL - Pharmacy Bulk Package - Ceftriaxone bag label
NDC 66288-6100-1 SMARxTPAK
CEFTRIAXONE FOR INJECTION, USP
PHARMACY BULK PACKAGE -
NOT FOR DIRECT INFUSION100 grams [ONE HUNDRED GRAMS] per Pharmacy Bulk Package Bag
FOR INTRAVENOUS USE ONLY
NOT TO BE DISPENSED AS A UNIT
Rx Only
Sterile, Nonpyrogenic, Preservative-Free
Each 100 gram Pharmacy Bulk Package contains sterile ceftriaxone sodium equivalent to 100 grams of ceftriaxone. The sodium content is 83 mg (3.6 mEq) per gram of ceftriaxone activity.
Use this formulation only in patients who require a 1 gram dose. Contains 100 doses (1 gram per dose). See package insert. USUAL DOSAGE: See package insert.
Prior to reconstitution, store dry powder at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature] Protect from light. After reconstitution, dilute and use promptly. DISCARD BAG WITHIN 4 HOURS AFTER INITIAL ENTRY. See package insert for full information.
THIS PHARMACY BULK PACKAGE IS INTENDED FOR PREPARING MANY SINGLE DOSES IN A PHARMACY ADMIXTURE PROGRAM.
Approximate Concentration Amount of Sterile Water for
Injection1 gram/10 mL 950 mL See package insert for detailed reconstitution, final dilution, and administration instructions.
PROTECT FROM LIGHT. THE INNER BAG SHOULD BE RETAINED IN THE OUTER BAG UNTIL TIME OF USE.
SmartPak® system components are not made with natural rubber latex.
Manufactured for
SAMSON
MEDICAL TECHNOLOGIES L.L.C.
Cherry Hill, NJ 08003,Date entered Time of entry Discard within 4 hours 
-
INGREDIENTS AND APPEARANCE
CEFTRIAXONE
ceftriaxone sodium injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66288-6100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFTRIAXONE SODIUM (UNII: 023Z5BR09K) (CEFTRIAXONE - UNII:75J73V1629) CEFTRIAXONE 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66288-6100-1 1 in 1 BAG; Type 0: Not a Combination Product 06/30/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090057 06/30/2014 Labeler - Samson Medical Technologies, LLC (102837429)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.