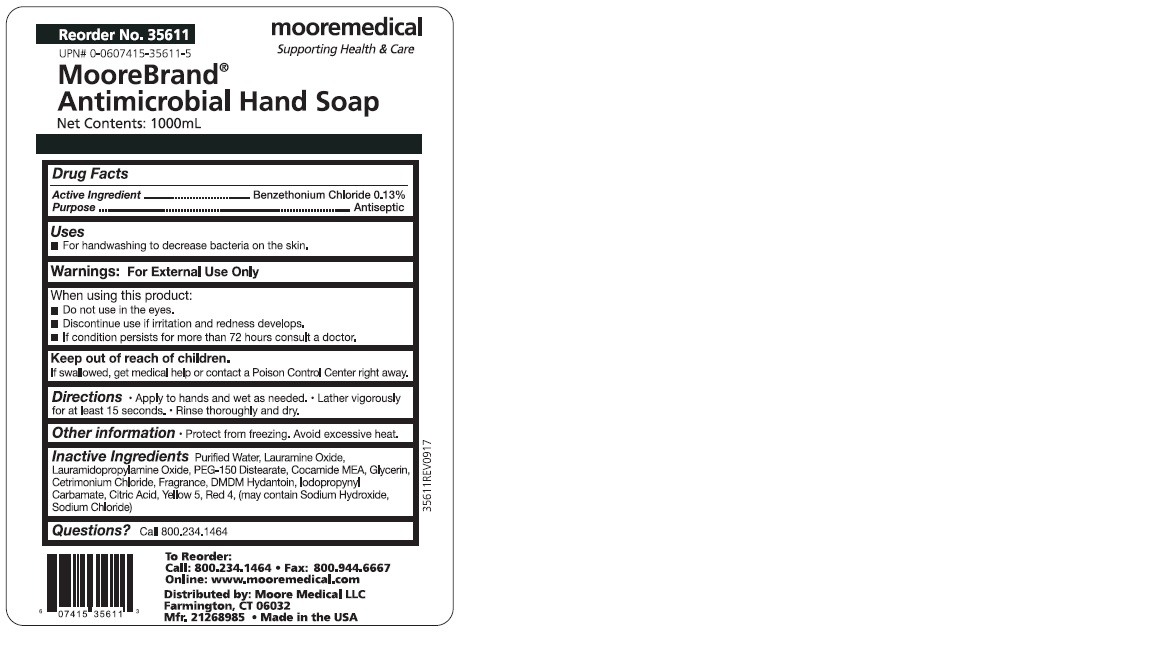
Antiseptic by Moore Medical
Antiseptic by
Drug Labeling and Warnings
Antiseptic by is a Otc medication manufactured, distributed, or labeled by Moore Medical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC- benzethonium chloride soap
Moore Medical
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Discontinue use if irritation and redness develops.
If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Direction:
- Apply to hands and wet as needed.
- Lather vigorously for at least 15 seconds.
- Rinse thoroughly and dry.
Purified Water, Lauramine Oxide, Lauramidopropylamine Oxide, PEG-150 Distearate,
Cocamide MEA, Glycerin, Cetrimonium Chloride, Fragrance, DMDM Hydantoin,
Iodopropynyl Carbamate, Citric Acid, Yellow 5, Red 4
Moore Medical
Supporting Health and Care
Reorder No. 35609
UPN# 0-0607415-35609-1
MooreBrand
Antimicrobial Hand Soap
Net Contents: 18oz (532mL)

| ANTISEPTIC
benzethonium chloride soap |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Moore Medical (051420107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Central Solutions | 007118524 | manufacture(55670-803) | |