POLYETHYLENE GLYCOL 3350 powder, for solution
Polyethylene Glycol 3350 by
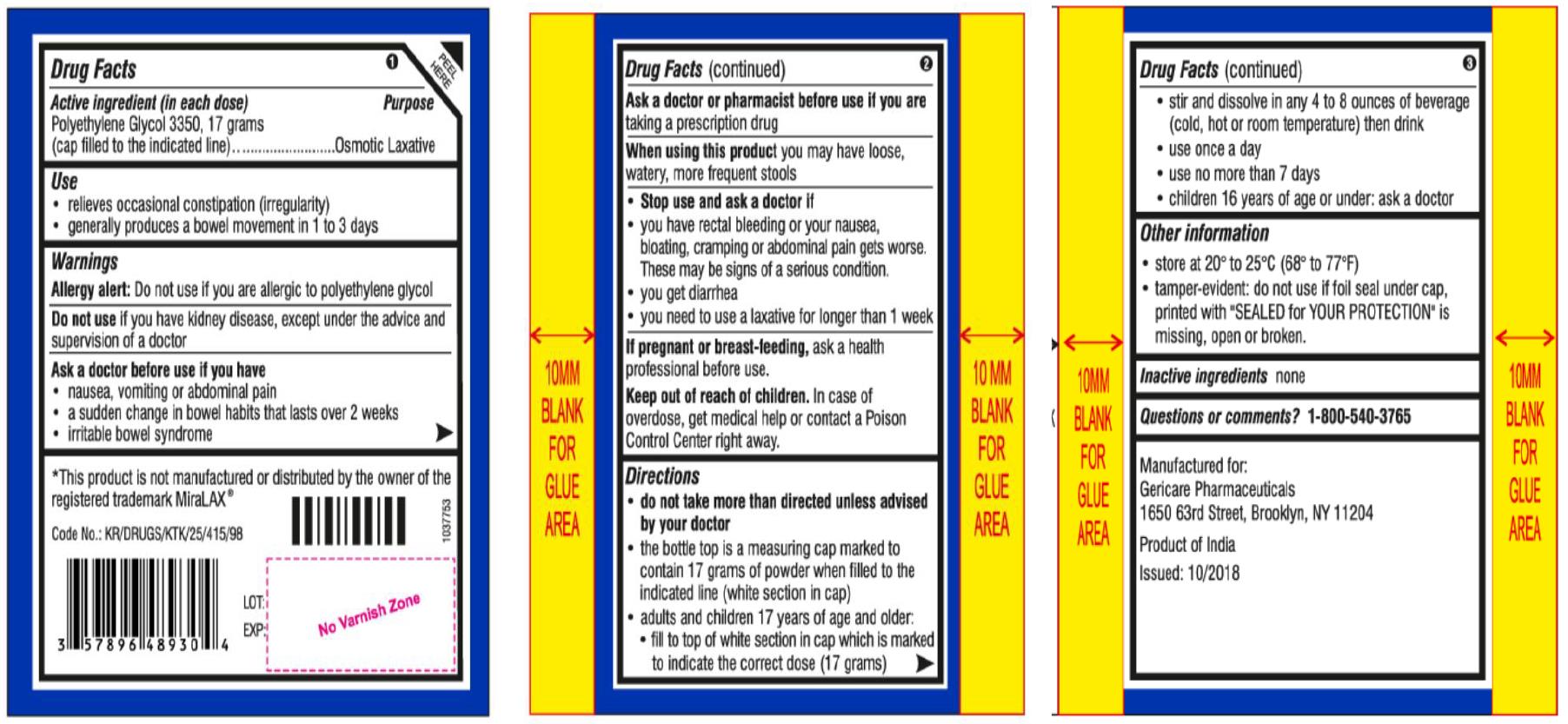
Drug Labeling and Warnings
Polyethylene Glycol 3350 by is a Otc medication manufactured, distributed, or labeled by Gericare Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED
- OTC - ACTIVE INGREDIENT SECTION
- OTC - PURPOSE
- USE
-
WARNINGS
OTC - DO NOT USE
Do not use if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- nausea, vomiting or abdominal pain
- a sudden change in bowel habits that lasts over 2 weeks
- irritable bowel syndrome
OTC - ASK DOCTOR/PHARMACIST
Ask a doctor of pharmacist before use if you are taking a prescription drug
Stop use and ask a doctor if
- you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
- you get diarrhea
- you need to use a laxative for longer than 1 week
-
Directions
- do not take more than directed unless advised by your doctor
- the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
- adults and children 17 years of age and older:
- fill to top of white section in cap which is marked to indicate the correct dose (17 g)
- stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
- use once a day
- use no more than 7 days
- children 16 years of age or under: ask a doctor
- Other Information
- INACTIVE INGREDIENT
- OTC - QUESTIONS
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 57896-490-30
Original
Prescription
Strength
Polyethylene Glycol 3350
Powder for Solution
Osmotic Laxative
- Relieves Occasional Constipation (Irregularity)
- Softens Stool
TAMPER-EVIDENT: DO NOT USE IF PRINTED FOIL SEAL UNDER CAP IS MISSING, OPEN OR BROKEN
- Dissolves in any beverage
- Sugar Free
NET WT 17.9 OZ (510 g)

-
INGREDIENTS AND APPEARANCE
POLYETHYLENE GLYCOL 3350
polyethylene glycol 3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57896-490 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color WHITE (Colorless upon dissolution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57896-490-30 510 g in 1 CAN; Type 0: Not a Combination Product 05/21/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203928 05/21/2019 Labeler - Gericare Pharmaceuticals (611196254) Establishment Name Address ID/FEI Business Operations Strides Pharma Science Limited 918513263 ANALYSIS(57896-490) , MANUFACTURE(57896-490) , PACK(57896-490)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.