BLADDER RELIEF (causticum, natrum muriaticum, nux vomica, pulsatilla- vulgaris, sepia, staphysagria, thuja occidentalis tablet
Bladder Relief by
Drug Labeling and Warnings
Bladder Relief by is a Homeopathic medication manufactured, distributed, or labeled by Magni Company, Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away
Stop use and ask a doctor if symptoms persist for more than 7 days or worsen, or if you have symptoms of a urinary tract infection
-
OTHER INFORMATION:
Store tightly closed in a cool, dry place (59° - 86°F)
Tamper sealed: Sealed for your protection. Do not use if seal is broken or missing
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopeia of the United
States
These statements are supported by the traditional homeopathic principles
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- INDICATIONS:
- QUESTIONS:
-
PACKAGE LABEL DISPLAY:
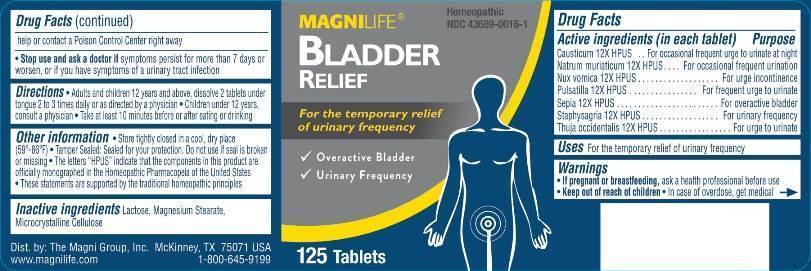
MAGNILIFE Homeopathic
BLADDER NDC: 43689-0016-1
RELIEF
For the temporary relief
of urinary frequency
√ Overactive Bladder
√ Urinary Frequency
125 Tablets

-
INGREDIENTS AND APPEARANCE
BLADDER RELIEF
causticum, natrum muriaticum, nux vomica, pulsatilla (vulgaris), sepia, staphysagria, thuja occidentalis tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43689-0016 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 12 [hp_X] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 12 [hp_X] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 12 [hp_X] THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 12 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape ROUND (Round convex) Size 6mm Flavor Imprint Code ML Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43689-0016-2 1 in 1 PACKAGE 1 NDC: 43689-0016-1 125 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/12/2015 Labeler - Magni Company (113501902) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43689-0016) , api manufacture(43689-0016) , label(43689-0016) , pack(43689-0016)
Trademark Results [Bladder Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLADDER RELIEF 86813107 not registered Dead/Abandoned |
The Magni Group, Inc. 2015-11-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.