KAISASA TATTOO NUMBING by Shenzhen Chengpinhui Technology Trading Co., Ltd.
KAISASA TATTOO NUMBING by
Drug Labeling and Warnings
KAISASA TATTOO NUMBING by is a Otc medication manufactured, distributed, or labeled by Shenzhen Chengpinhui Technology Trading Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
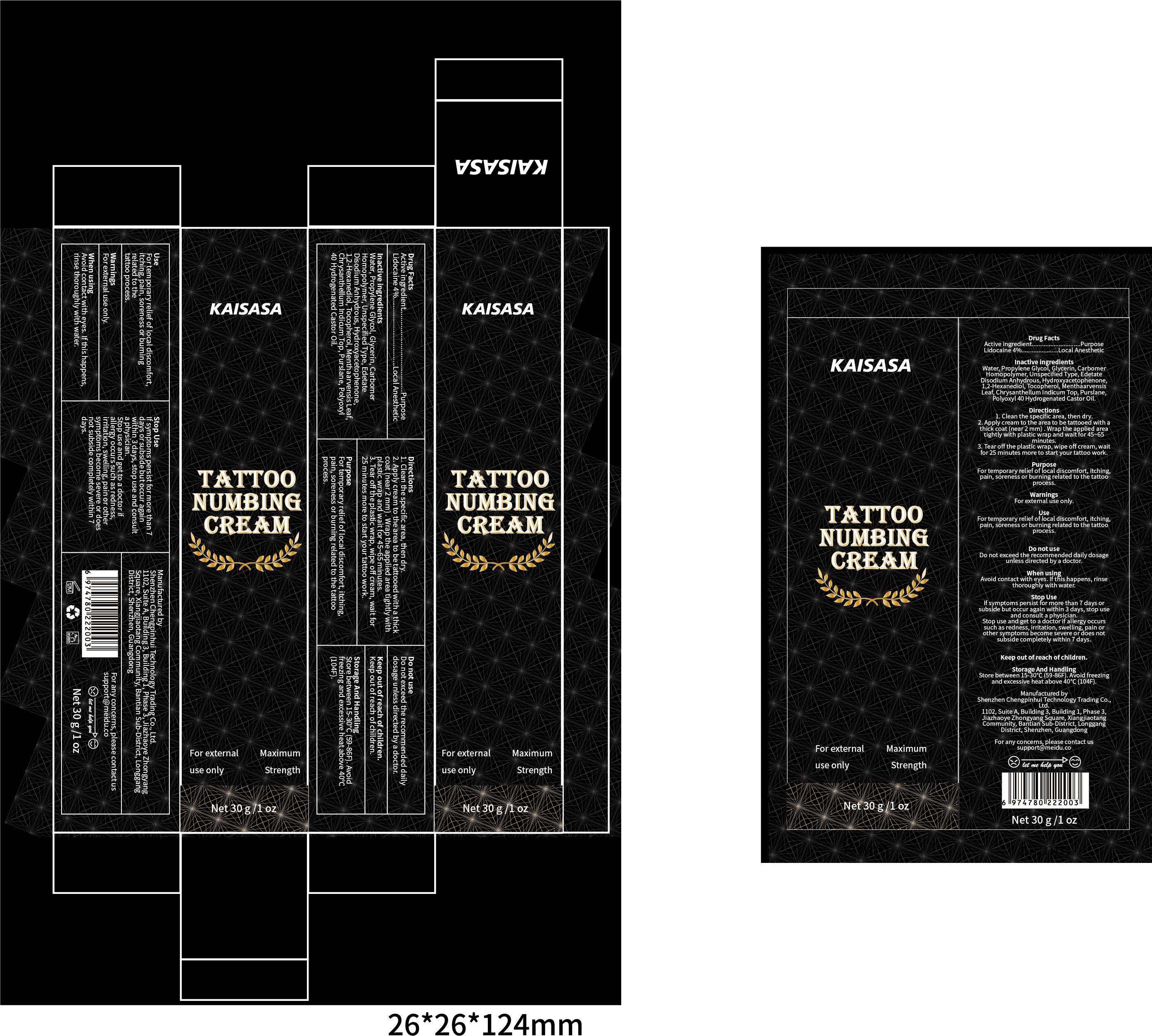
KAISASA TATTOO NUMBING- lidocaine cream
Shenzhen Chengpinhui Technology Trading Co., Ltd.
----------
Purpose
For temporary reliefof local discomfort, itching:pain, soreness or burning related to the tattoo process.
Use
For temporary relief of local discomfort,itching, pain, soreness or bumingrelated to thetattoo process
Stop use
lf symptoms persist for more than 7days or subside but occur againwithin 3 days, stop use and consulta physician.Stop use and get to a doctor ifallergy occurs such as rednessirritation, swelling, pain or othersymptoms become severe or doesnot subside completely within 7days.
Directions
1. clean the specific area, then dry.
2. Apply cream to the area to be tattooed with a thickcoat (near 2 mm),Wrap the applied area tightly withplastic wrap and wait for 45~65 minutes.
3. Tear off the plastic wrap, wipe off cream, wait for25 minutes more to start your tattoo work.
Storage and Handling:
Store between 15-30°C(59-86F).Avoid freezing and excessive heat above 40°C(104F)
| KAISASA TATTOO NUMBING
lidocaine cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Shenzhen Chengpinhui Technology Trading Co., Ltd. (722827072) |
| Registrant - Shenzhen Chengpinhui Technology Trading Co., Ltd. (722827072) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Chengpinhui Technology Trading Co., Ltd. | 722827072 | manufacture(85355-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.