TIROSINT SOL- levothyroxine sodium solution
Tirosint by
Drug Labeling and Warnings
Tirosint by is a Prescription medication manufactured, distributed, or labeled by IBSA Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TIROSINT®-SOL safely and effectively. See full prescribing information for TIROSINT®-SOL.
TIROSINT®-SOL (levothyroxine sodium) oral solution
Initial U.S. Approval: 2000WARNING: NOT FOR TREATMENT OF OBESITY or FOR WEIGHT LOSS
See full prescribing information for complete boxed warning
INDICATIONS AND USAGE
TIROSINT-SOL is L-thyroxine (T4) indicated for:
- Hypothyroidism - As replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism (1)
- Pituitary Thyrotropin (Thyroid-timulating Hormone, TSH) Suppression - As an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent well-differentiated thyroid cancer (1)
Limitations of Use:
DOSAGE AND ADMINISTRATION
- Administer once daily, on an empty stomach, one-half to one hour before breakfast (2.1)
- Administer at least 4 hours before or after drugs that are known to interfere with absorption (2.1)
- Evaluate the need for dose adjustments when regularly administering within an hour of certain foods that may affect TIROSINT-SOL absorption (2.1)
- To administer TIROSINT-SOL in water, squeeze the contents of one single unit-dose ampule into a glass or cup containing water (2.1)
- To administer TIROSINT-SOL directly, either squeeze it into the mouth OR onto a spoon and immediately consume (2.1)
- Starting dose depends on a variety of factors, including age, body weight, cardiovascular status, concomitant medical conditions (including pregnancy), concomitant medications, co-administered food, and the specific nature of the condition being treated. Peak therapeutic effect may not be attained for 4-6 weeks (2.2)
- See full prescribing information for dosing in specific patient populations (2.3)
- Adequacy of therapy determined with periodic monitoring of TSH and/or T4 as well as clinical status (2.4)
DOSAGE FORMS AND STRENGTHS
Oral solution: 13, 25, 50, 75, 88, 100, 112, 125, 137, 150, 175, 200 mcg/mL (3)
WARNINGS AND PRECAUTIONS
- Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate TIROSINT-SOL at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation. (2.3, 5.1, 8.5)
- Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma. (5.2)
- Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of TIROSINT-SOL treatment. (5.3)
- Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism. (5.4)
- Worsening of diabetic control: therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy. (5.5)
- Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose. (5.6)
ADVERSE REACTIONS
Adverse reactions associated with TIROSINT-SOL are primarily those of hyperthyroidism due to therapeutic overdosage including: arrhythmias, myocardial infarction, dyspnea, muscle spasm, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash (6)
To report SUSPECTED ADVERSE REACTIONS, contact IBSA Pharma Inc. at 1-800-587-3513, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to TIROSINT-SOL (7)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: NOT FOR TREATMENT OF OBESITY or FOR WEIGHT LOSS
1 INDICATION AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Information
2.2 General Principles of Dosing
2.3 Dosing in Specific Patient Populations
2.4 Monitoring TSH and/or Thyroxine (T4) Levels
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
5.2 Myxedema Coma
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
5.5 Worsening of Diabetic Control
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-Replacement
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
7.2 Antidiabetic Therapy
7.3 Oral Anticoagulants
7.4 Digitalis Glycosides
7.5 Antidepressant Therapy
7.6 Ketamine
7.7 Sympathomimetics
7.8 Tyrosine-Kinase Inhibitors
7.9 Drug-Food Interactions
7.10 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR TREATMENT OF OBESITY or FOR WEIGHT LOSS
- Thyroid hormones, including TIROSINT-SOL, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss.
- In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction.
- Larger doses may produce serious or even life threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects [see Adverse Reactions (6), Drug Interactions (7.7), and Overdosage (10)].
-
1 INDICATION AND USAGE
Hypothyroidism
TIROSINT-SOL is indicated as a replacement therapy in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Pituitary Thyrotropin (ThyroidStimulating Hormone, TSH) Suppression
TIROSINT-SOL is indicated as an adjunct to surgery and radioiodine therapy in the management of thyrotropin-dependent-well-differentiated thyroid cancer.
Limitations of Use:
- TIROSINT-SOL is not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients as there are no clinical benefits and overtreatment with TIROSINT-SOL may induce hyperthyroidism [see Warnings and Precautions (5.4)].
- TIROSINT-SOL is not indicated for treatment of transient hypothyroidism during the recovery phase of subacute thyroiditis.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Information
Administer TIROSINT-SOL as a single daily oral dose, on an empty stomach, one-half to one hour before breakfast.
Administer TIROSINT-SOL at least 4 hours before or after drugs known to interfere with TIROSINT-SOL absorption [see Drug Interactions (7.1)].
Evaluate the need for dose adjustments when regularly administering within an hour of certain foods that may affect TIROSINT-SOL absorption [see Drug Interactions (7.9) and Clinical Pharmacology (12.3)]. TIROSINT-SOL may be administered in water or directly into the mouth:
- To administer TIROSINT-SOL in water, squeeze the contents of one single unit-dose ampule into a glass or cup containing water. Stir the diluted TIROSINT-SOL and drink all of it immediately. Rinse the glass or cup with additional water and drink the contents to ensure that the total dose is taken. Do not dilute TIROSINT-SOL in a medium other than water. Open the ampule and prepare the solution immediately before intake.
- To administer TIROSINT-SOL directly (without water), either squeeze it into the mouth OR onto a spoon and immediately consume.
2.2 General Principles of Dosing
The dose of TIROSINT-SOL for hypothyroidism or pituitary TSH suppression depends on a variety of factors including: the patient's age, body weight, cardiovascular status, concomitant medical conditions (including pregnancy), concomitant medications, coadministered food, and the specific nature of the condition being treated [see Dosage and Administration (2.3), Warnings and Precautions (5), and Drug Interactions (7)]. Dosing must be individualized to account for these factors and dose adjustments made based on periodic assessment of the patient's clinical response and laboratory parameters [see Dosage and Administration (2.4)].
The peak therapeutic effect of a given dose of TIROSINT-SOL may not be attained for 4 to 6 weeks.
2.3 Dosing in Specific Patient Populations
Primary Hypothyroidism in Adults and in Adolescents in Whom Growth and Puberty are Complete
Start TIROSINT-SOL at the full replacement dose in otherwise healthy, non-elderly individuals who have been hypothyroid for only a short time (such as a few months). The average full replacement dose of TIROSINT-SOL is approximately 1.6 mcg per kg per day (for example: 100 to 125 mcg per day for a 70 kg adult).
Adjust the dose by 12.5 to 25 mcg increments every 4 to 6 weeks until the patient is clinically euthyroid and the serum TSH returns to normal. Doses greater than 200 mcg per day are seldom required. An inadequate response to daily doses greater than 300 mcg per day is rare and may indicate poor compliance, malabsorption, drug interactions, or a combination of these factors.
For elderly patients or patients with underlying cardiovascular disease, start with a dose of 12.5 to 25 mcg per day. Increase the dose every 6 to 8 weeks, as needed, until the patient is clinically euthyroid and the serum TSH returns to normal. The full replacement dose of TIROSINT-SOL may be less than 1 mcg per kg per day in elderly patients.
In patients with severe longstanding hypothyroidism, start with a dose of 12.5 to 25 mcg per day. Adjust the dose in 12.5 to 25 mcg increments every 2 to 4 weeks until the patient is clinically euthyroid and the serum TSH level is normalized.
Secondary or Tertiary Hypothyroidism
Start TIROSINT-SOL at the full replacement dose in otherwise healthy, non-elderly individuals. Start with a lower dose in elderly patients with underlying cardiovascular disease or patients with severe longstanding hypothyroidism as described above. Serum TSH is not a reliable measure of TIROSINT-SOL dose adequacy in patients with secondary or tertiary hypothyroidism and should not be used to monitor therapy. Use the serum free-T4 level to monitor adequacy of therapy in this patient population. Titrate TIROSINT-SOL dosing per above instructions until the patient is clinically euthyroid and the serum free-T4 level is restored to the upper half of the normal range.
Pediatric Dosage - Congenital or Acquired Hypothyroidism
The recommended daily dose of TIROSINT-SOL in pediatric patients with hypothyroidism is based on body weight and changes with age as described in Table 1. Start TIROSINT-SOL at the full daily dose in most pediatric patients. Start at a lower dose in newborns (0 to 3 months) at risk for cardiac failure and children at risk for hyperactivity (see below). Monitor for clinical and laboratory response [see Dosage and Administration (2.4)].
Table 1: TIROSINT-SOL Dosing Guidelines for Pediatric Hypothyroidism AGE Daily Dose Per Kg Body Weight* - * The dose should be adjusted based on clinical response and laboratory parameters [see Dosage and Administration (2.4) and Use in Specific Populations (8.4)].
0-3 months 10-15 mcg/kg/day 3-6 months 8-10 mcg/kg/day 6-12 months 6-8 mcg/kg/day 1-5 years 5-6 mcg/kg/day 6-12 years 4-5 mcg/kg/day Greater than 12 years but growth and puberty incomplete 2-3 mcg/kg/day Growth and puberty complete 1.6 mcg/kg/day Pregnancy
Preexisting Hypothyroidism: TIROSINT-SOL dose requirements may increase during pregnancy. Measure serum TSH and free-T4 as soon as pregnancy is confirmed and, at a minimum, during each trimester of pregnancy. In patients with primary hypothyroidism, maintain serum TSH in the trimester-specific reference range. For patients with serum TSH above the normal trimester specific range, increase the dose of TIROSINT-SOL by 12.5 to 25 mcg per day and measure TSH every 4 weeks until a stable TIROSINT-SOL dose is reached and serum TSH is within the normal trimester specific range. Reduce TIROSINT-SOL dosage to pre-pregnancy levels immediately after delivery and measure serum TSH levels 4 to 8 weeks postpartum to ensure the TIROSINT-SOL dose is appropriate.
New Onset Hypothyroidism: Normalize thyroid function as rapidly as possible. In patients with moderate to severe signs and symptoms of hypothyroidism, start TIROSINT-SOL at the full replacement dose (1.6 mcg per kg body weight per day). In patients with mild hypothyroidism (TSH < 10 mIU per Liter), start TIROSINT-SOL at 1.0 mcg per kg body weight per day. Evaluate serum TSH every 4 weeks and adjust TIROSINT-SOL dosage until serum TSH is within the normal trimester specific range [see Use in Specific Populations (8.1)].
2.4 Monitoring TSH and/or Thyroxine (T4) Levels
Assess the adequacy of therapy by periodic assessment of laboratory tests and clinical evaluation. Persistent clinical and laboratory evidence of hypothyroidism, despite an apparent adequate replacement dose of TIROSINT-SOL, may be evidence of inadequate absorption, poor compliance, drug interactions, or a combination of these factors.
Adults
In adult patients with primary hypothyroidism, monitor serum TSH levels after an interval of 6 to 8 weeks after any change in dose. In patients on a stable and appropriate replacement dose, evaluate clinical and biochemical response every 6 to 12 months and whenever there is a change in the patient's clinical status.
Pediatrics
In patients with congenital hypothyroidism, assess the adequacy of replacement therapy by measuring both serum TSH and total or free-T4. Monitor TSH and total or free-T4 in children as follows: 2 and 4 weeks after the initiation of treatment, 2 weeks after any change in dosage, and then every 3 to 12 months thereafter following dose stabilization until growth is completed. Poor compliance or abnormal values may necessitate more frequent monitoring. Perform routine clinical examination, including assessment of mental and physical growth and development, and bone maturation, at regular intervals. While the general aim of therapy is to normalize the serum TSH level, TSH may not normalize in some patients due to in utero hypothyroidism causing a resetting of pituitary-thyroid feedback. Failure of the serum T4 to increase into the upper half of the normal range within 2 weeks of initiation of TIROSINT-SOL therapy and/or of the serum TSH to decrease below 20 mIU per Liter within 4 weeks may indicate the child is not receiving adequate therapy. Assess compliance, dose of medication administered, and method of administration prior to increasing the dose of TIROSINT-SOL [see Warnings and Precautions (5.4) and Use in Specific Populations (8.4)].
-
3 DOSAGE FORMS AND STRENGTHS
TIROSINT-SOL oral solution is a clear, colorless to slightly yellow solution supplied in a 1 mL white, non-transparent, unit-dose ampule. Each ampule bears a colored label with the dosage strength and the product name (TIROSINT-SOL):
Strength (mcg/mL) Color 13 Green 25 Orange 50 White 75 Purple 88 Olive 100 Yellow 112 Rose 125 Brown 137 Turquoise 150 Blue 175 Lilac 200 Pink -
4 CONTRAINDICATIONS
TIROSINT-SOL is contraindicated in patients with:
- hypersensitivity to glycerol, the inactive ingredient in TIROSINT-SOL [see Adverse Events (6)].
- Uncorrected adrenal insufficiency [see Warnings and Precautions (5.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiac Adverse Reactions in the Elderly and in Patients with Underlying Cardiovascular Disease
Overtreatment with levothyroxine may cause an increase in heart rate, cardiac wall thickness, and cardiac contractility, and may precipitate angina or arrhythmias, particularly in patients with cardiovascular disease and in elderly patients. Initiate TIROSINT-SOL therapy in this population at lower doses than those recommended in younger individuals or in patients without cardiac disease [see Dosage and Administration (2.3) and Use in Specific Populations (8.5)].
Monitor for cardiac arrhythmias during surgical procedures in patients with coronary artery disease receiving suppressive TIROSINT-SOL therapy. Monitor patients receiving concomitant TIROSINT-SOL and sympathomimetic agents for signs and symptoms of coronary insufficiency. If cardiac symptoms develop or worsen, reduce the TIROSINT-SOL dose or withhold it for one week and restart at a lower dose.
5.2 Myxedema Coma
Myxedema coma is a life-threatening emergency characterized by poor circulation and hypometabolism, and may result in unpredictable absorption of levothyroxine sodium from the gastrointestinal tract. Use of oral thyroid hormone drug products is not recommended to treat myxedema coma. Administer thyroid hormone products formulated for intravenous administration to treat myxedema coma.
5.3 Acute Adrenal Crisis in Patients with Concomitant Adrenal Insufficiency
Thyroid hormone increases metabolic clearance of glucocorticoids. Initiation of thyroid hormone therapy prior to initiating glucocorticoid therapy may precipitate an acute adrenal crisis in patients with adrenal insufficiency. Treat patients with adrenal insufficiency with replacement glucocorticoids prior to initiating treatment with TIROSINT-SOL [see Contraindications (4)].
5.4 Prevention of Hyperthyroidism or Incomplete Treatment of Hypothyroidism
TIROSINT-SOL has a narrow therapeutic index. Over- or under-treatment with TIROSINT-SOL may have negative effects on growth and development, cardiovascular function, bone metabolism, reproductive function, cognitive function, emotional state, gastrointestinal function, and on glucose and lipid metabolism. Titrate the dose of TIROSINT-SOL carefully and monitor response to titration to avoid these effects [see Dosage and Administration (2.4)]. Monitor for the presence of drug or food interactions when using TIROSINT-SOL and adjust the dose as necessary [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
5.5 Worsening of Diabetic Control
Addition of levothyroxine therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy [see Drug Interactions (7.2)].
5.6 Decreased Bone Mineral Density Associated with Thyroid Hormone Over-Replacement
Increased bone resorption and decreased bone mineral density may occur as a result of levothyroxine over-replacement, particularly in post-menopausal women. The increased bone resorption may be associated with increased serum levels and urinary excretion of calcium and phosphorous, elevations in bone alkaline phosphatase, and suppressed serum parathyroid hormone levels. Administer the minimum dose of TIROSINT-SOL that achieves the desired clinical and biochemical response to mitigate this risk.
-
6 ADVERSE REACTIONS
Adverse reactions associated with TIROSINT-SOL therapy are primarily those of hyperthyroidism due to therapeutic overdosage [see Warnings and Precautions (5) and Overdosage (10)]. They include the following:
- General: fatigue, increased appetite, weight loss, heat intolerance, fever, excessive sweating
- Central nervous system: headache, hyperactivity, nervousness, anxiety, irritability, emotional lability, insomnia
- Musculoskeletal: tremors, muscle weakness, muscle spasm
- Cardiovascular: palpitations, tachycardia, arrhythmias, increased pulse and blood pressure, heart failure, angina, myocardial infarction, cardiac arrest
- Respiratory: dyspnea
- Gastrointestinal (GI): diarrhea, vomiting, abdominal cramps, elevations in liver function tests
- Dermatologic: hair loss, flushing, rash
- Endocrine: decreased bone mineral density
- Reproductive: menstrual irregularities, impaired fertility
Seizures have been reported rarely with the institution of levothyroxine therapy.
Adverse Reactions in Children
Pseudotumor cerebri and slipped capital femoral epiphysis have been reported in children receiving levothyroxine therapy. Overtreatment may result in craniosynostosis in infants and premature closure of the epiphyses in children with resultant compromised adult height.
Hypersensitivity Reactions
Hypersensitivity reactions to inactive ingredients have occurred in patients treated with thyroid hormone products. These include urticaria, pruritus, skin rash, flushing, angioedema, various GI symptoms (abdominal pain, nausea, vomiting and diarrhea), fever, arthralgia, serum sickness and wheezing. Hypersensitivity to levothyroxine itself is not known to occur.
-
7 DRUG INTERACTIONS
7.1 Drugs Known to Affect Thyroid Hormone Pharmacokinetics
Many drugs can exert effects on thyroid hormone pharmacokinetics (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to TIROSINT-SOL (see Tables 2 - 5 below).
Table 2: Drugs That May Decrease T4 Absorption (Hypothyroidism) Potential impact: Concurrent use may reduce the efficacy of TIROSINT-SOL by binding and delaying or preventing absorption, potentially resulting in hypothyroidism. Drug or Drug Class Effect Calcium Carbonate
Ferrous SulfateCalcium carbonate may form an insoluble chelate with levothyroxine, and ferrous sulfate likely forms a ferric-thyroxine complex. Administer TIROSINT-SOL at least 4 hours apart from these agents. Orlistat Monitor patients treated concomitantly with orlistat and TIROSINT-SOL for changes in thyroid function. Bile Acid Sequestrants
-Colesevelam
-Cholestyramine
-Colestipol
Ion Exchange Resins
-Kayexalate
-SevelamerBile acid sequestrants and ion exchange resins are known to decrease levothyroxine absorption. Administer TIROSINT-SOL at least 4 hours prior to these drugs or monitor thyrotropin (TSH) levels. Other drugs:
Proton Pump Inhibitors
Sucralfate
Antacids
- Aluminum & Magnesium Hydroxides
- SimethiconeGastric acidity is an essential requirement for adequate absorption of levothyroxine. Sucralfate, antacids and proton pump inhibitors may cause hypochlorhydria, affect intragastric pH, and reduce levothyroxine absorption. Monitor patients appropriately. Table 3: Drugs That May Alter T4 and Triiodothyronine (T3) Serum Transport Without Affecting Free Thyroxine (FT4) Concentration (Euthyroidism) Drug or Drug Class Effect Clofibrate
Estrogen-containing oral contraceptives
Estrogens (oral)
Heroin / Methadone
5-Fluorouracil
Mitotane
TamoxifenThese drugs may increase serum thyroxine-binding globulin (TBG) concentration. Androgens / Anabolic Steroids
Asparaginase
Glucocorticoids
Slow-Release Nicotinic AcidThese drugs may decrease serum TBG concentration. Potential impact (below): Administration of these agents with TIROSINT-SOL results in an initial transient increase in FT4. Continued administration results in a decrease in serum T4 and normal FT4 and TSH concentrations. Salicylates (> 2 g/day) Salicylates inhibit binding of T4 and T3 to TBG and transthyretin. An initial increase in serum FT4 is followed by return of FT4 to normal levels with sustained therapeutic serum salicylate concentrations, although total T4 levels may decrease by as much as 30%. Other drugs:
Carbamazepine
Furosemide (> 80 mg IV)
Heparin
Hydantoins
Non-Steroidal Anti-inflammatory Drugs
- FenamatesThese drugs may cause protein-binding site displacement. Furosemide has been shown to inhibit the protein binding of T4 to TBG and albumin, causing an increased free-T4 fraction in serum. Furosemide competes for T4-binding sites on TBG, prealbumin, and albumin, so that a single high dose can acutely lower the total T4 level. Phenytoin and carbamazepine reduce serum protein binding of levothyroxine, and total and free-T4 may be reduced by 20% to 40%, but most patients have normal serum TSH levels and are clinically euthyroid. Closely monitor thyroid hormone parameters. Table 4: Drugs That May Alter Hepatic Metabolism of T4 (Hypothyroidism) Potential impact: Stimulation of hepatic microsomal drug-metabolizing enzyme activity may cause increased hepatic degradation of levothyroxine, resulting in increased TIROSINT-SOL requirements. Drug or Drug Class Effect Phenobarbital
RifampinPhenobarbital has been shown to reduce the response to thyroxine. Phenobarbital increases L-thyroxine metabolism by inducing uridine 5'-diphospho-glucuronosyltransferase (UGT) and leads to a lower T4 serum levels. Changes in thyroid status may occur if barbiturates are added or withdrawn from patients being treated for hypothyroidism. Rifampin has been shown to accelerate the metabolism of levothyroxine. Table 5: Drugs That May Decrease Conversion of T4 to T3 Potential impact: Administration of these enzyme inhibitors decreases the peripheral conversion of T4 to T3, leading to decreased T3 levels. However, serum T4 levels are usually normal but may occasionally be slightly increased. Drug or Drug Class Effect Beta-adrenergic antagonists
(e.g., Propranolol > 160 mg/day)In patients treated with large doses of propranolol (> 160 mg/day), T3 and T4 levels change, TSH levels remain normal, and patients are clinically euthyroid. Actions of particular beta-adrenergic antagonists may be impaired when the hypothyroid patient is converted to the euthyroid state. Glucocorticoids
(e.g., Dexamethasone ≥ 4 mg/day)Short-term administration of large doses of glucocorticoids may decrease serum T3 concentrations by 30% with minimal change in serum T4 levels. However, long-term glucocorticoid therapy may result in slightly decreased T3 and T4 levels due to decreased TBG production (See Table 3 above). Other drugs:
AmiodaroneAmiodarone inhibits peripheral conversion of levothyroxine (T4) to triiodothyronine (T3) and may cause isolated biochemical changes (increase in serum free-T4, and decrease or normal free-T3) in clinically euthyroid patients. 7.2 Antidiabetic Therapy
Addition of TIROSINT-SOL therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control, especially when thyroid therapy is started, changed, or discontinued [see Warnings and Precautions (5.5)].
7.3 Oral Anticoagulants
TIROSINT-SOL increases the response to oral anticoagulant therapy. Therefore, a decrease in the dose of anticoagulant may be warranted with correction of the hypothyroid state or when the TIROSINT-SOL dose is increased. Closely monitor coagulation tests to permit appropriate and timely dosage adjustments.
7.4 Digitalis Glycosides
TIROSINT-SOL may reduce the therapeutic effects of digitalis glycosides. Serum digitalis glycoside levels may decrease when a hypothyroid patient becomes euthyroid, necessitating an increase in the dose of digitalis glycosides.
7.5 Antidepressant Therapy
Concurrent use of tricyclic (e.g., amitriptyline) or tetracyclic (e.g., maprotiline) antidepressants and TIROSINT-SOL may increase the therapeutic and toxic effects of both drugs, possibly due to increased receptor sensitivity to catecholamines. Toxic effects may include increased risk of cardiac arrhythmias and central nervous system stimulation. TIROSINT-SOL may accelerate the onset of action of tricyclics. Administration of sertraline in patients stabilized on TIROSINT-SOL may result in increased TIROSINT-SOL requirements.
7.6 Ketamine
Concurrent use of ketamine and TIROSINT-SOL may produce marked hypertension and tachycardia. Closely monitor blood pressure and heart rate in these patients.
7.7 Sympathomimetics
Concurrent use of sympathomimetics and TIROSINT-SOL may increase the effects of sympathomimetics or thyroid hormone. Thyroid hormones may increase the risk of coronary insufficiency when sympathomimetic agents are administered to patients with coronary artery disease.
7.8 Tyrosine-Kinase Inhibitors
Concurrent use of tyrosine-kinase inhibitors such as imatinib may cause hypothyroidism. Closely monitor TSH levels in such patients.
7.9 Drug-Food Interactions
Consumption of certain foods may affect TIROSINT-SOL absorption thereby necessitating adjustments in dosing [see Dosage and Administration (2.1)]. Soybean flour (infant formula), cottonseed meal, walnuts, and dietary fiber may bind and decrease the absorption of TIROSINT-SOL from the gastrointestinal tract. Grapefruit juice may delay the absorption of levothyroxine and reduce its bioavailability.
7.10 Drug-Laboratory Test Interactions
Consider changes in TBG concentration when interpreting T4 and T3 values. Measure and evaluate unbound (free) hormone and/or determine the free-T4 index (FT4I) in this circumstance. Pregnancy, infectious hepatitis, estrogens, estrogencontaining oral contraceptives, and acute intermittent porphyria increase TBG concentrations. Nephrosis, severe hypoproteinemia, severe liver disease, acromegaly, androgens and corticosteroids decrease TBG concentration. Familial hyper or hypothyroxine binding globulinemias have been described, with the incidence of TBG deficiency approximating 1 in 9000.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Experience with levothyroxine use in pregnant women, including data from post-marketing studies, have not reported increased rates of major birth defects or miscarriages [see Data]. There are risks to the mother and fetus associated with untreated hypothyroidism in pregnancy. Since thyroid-stimulating hormone (TSH) levels may increase during pregnancy, TSH should be monitored and TIROSINT-SOL dosage adjusted during pregnancy [see Clinical Considerations]. There are no animal studies conducted with levothyroxine during pregnancy. TIROSINT-SOL should not be discontinued during pregnancy and hypothyroidism diagnosed during pregnancy should be promptly treated.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Maternal hypothyroidism during pregnancy is associated with a higher rate of complications, including spontaneous abortion, gestational hypertension, preeclampsia, stillbirth, and premature delivery. Untreated maternal hypothyroidism may have an adverse effect on fetal neurocognitive development.
Dose Adjustments During Pregnancy and the Postpartum Period
Pregnancy may increase TIROSINT-SOL requirements. Serum TSH levels should be monitored and the TIROSINT-SOL dosage adjusted during pregnancy. Since postpartum TSH levels are similar to preconception values, the TIROSINT-SOL dosage should return to the pre-pregnancy dose immediately after delivery [see Dosage and Administration (2.3)].
Data
Human Data
Levothyroxine is approved for use as a replacement therapy for hypothyroidism. There is a long experience of levothyroxine use in pregnant women, including data from post-marketing studies that have not reported increased rates of fetal malformations, miscarriages or other adverse maternal or fetal outcomes associated with levothyroxine use in pregnant women.
8.2 Lactation
Risk Summary
Limited published studies report that levothyroxine is present in human milk. However, there is insufficient information to determine the effects of levothyroxine on the breastfed infant and no available information on the effects of levothyroxine on milk production. Adequate levothyroxine treatment during lactation may normalize milk production in hypothyroid lactating mothers. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TIROSINT-SOL and any potential adverse effects on the breastfed infant from TIROSINT-SOL or from the underlying maternal condition.
8.4 Pediatric Use
The initial dose of TIROSINT-SOL varies with age and body weight. Dosing adjustments are based on an assessment of the individual patient's clinical and laboratory parameters [see Dosage and Administration (2.3, 2.4)].
In children in whom a diagnosis of permanent hypothyroidism has not been established, discontinue TIROSINT-SOL administration for a trial period, but only after the child is at least 3 years of age. Obtain serum T4 and TSH levels at the end of the trial period, and use laboratory test results and clinical assessment to guide diagnosis and treatment, if warranted.
Congenital Hypothyroidism [see Dosage and Administration (2.3, 2.4)]
Rapid restoration of normal serum T4 concentrations is essential for preventing the adverse effects of congenital hypothyroidism on intellectual development as well as on overall physical growth and maturation. Therefore, initiate TIROSINT-SOL therapy immediately upon diagnosis. Levothyroxine is generally continued for life in these patients.
Closely monitor infants during the first two weeks of TIROSINT-SOL therapy for cardiac overload, arrhythmias, and aspiration from avid suckling.
Closely monitor patients to avoid undertreatment and overtreatment. Undertreatment may have deleterious effects on intellectual development and linear growth. Overtreatment has been associated with craniosynostosis in infants, and may adversely affect the tempo of brain maturation and accelerate the bone age with resultant premature closure of the epiphyses and compromised adult stature.
Acquired Hypothyroidism in Pediatric Patients
Closely monitor patients to avoid undertreatment and overtreatment. Undertreatment may result in poor school performance due to impaired concentration and slowed mentation and in reduced adult height. Overtreatment may accelerate the bone age and result in premature epiphyseal closure and compromised adult stature.
Treated children may manifest a period of catchup growth, which may be adequate in some cases to normalize adult height. In children with severe or prolonged hypothyroidism, catchup growth may not be adequate to normalize adult height.
8.5 Geriatric Use
Because of the increased prevalence of cardiovascular disease among the elderly, initiate TIROSINT-SOL at less than the full replacement dose [see Warnings and Precautions (5.1) and Dosage and Administration (2.3)]. Atrial arrhythmias can occur in elderly patients. Atrial fibrillation is the most common of the arrhythmias observed with levothyroxine overtreatment in the elderly.
-
10 OVERDOSAGE
The signs and symptoms of overdosage are those of hyperthyroidism [see Warnings and Precautions (5) and Adverse Reactions (6)]. In addition, confusion and disorientation may occur. Cerebral embolism, shock, coma, and death have been reported. Seizures occurred in a 3-year-old child ingesting 3.6 mg of levothyroxine. Symptoms may not necessarily be evident or may not appear until several days after ingestion of levothyroxine sodium.
Reduce the TIROSINT-SOL dose or discontinue temporarily if signs or symptoms of overdosage occur. Initiate appropriate supportive treatment as dictated by the patient's medical status.
For current information on the management of poisoning or overdosage, contact the National Poison Control Center at 1-800-222-1222 or www.poison.org.
-
11 DESCRIPTION
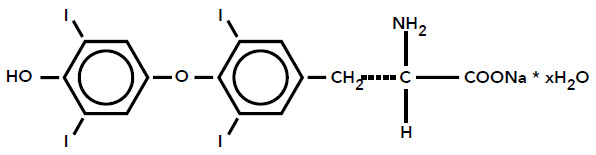
TIROSINT-SOL (levothyroxine sodium) oral solution contains synthetic L-3,3',5,5'tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C15H10I4NNaO4 ∙ x H2O (where x = 5), molecular weight of 798.86 g/mol (anhydrous), and structural formula as shown:
TIROSINT-SOL oral solution is a clear, colorless to slightly yellow solution supplied in a 1 mL white, non-transparent, unit-dose ampule and is available in the following strengths (mcg/mL): 13, 25, 50, 75, 88, 100, 112, 125, 137, 150, 175, 200.
The inactive ingredients in TIROSINT-SOL are glycerol and water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Thyroid hormones exert their physiologic actions through control of DNA transcription and protein synthesis. Triiodothyronine (T3) and L-thyroxine (T4) diffuse into the cell nucleus and bind to thyroid receptor proteins attached to DNA. This hormone nuclear receptor complex activates gene transcription and synthesis of messenger RNA and cytoplasmic proteins.
The physiological actions of thyroid hormones are produced predominantly by T3, the majority of which (approximately 80%) is derived from T4 by deiodination in peripheral tissues.
12.2 Pharmacodynamics
Oral levothyroxine sodium is a synthetic T4 hormone that exerts the same physiologic effect as endogenous T4, thereby maintaining normal T4 levels when a deficiency is present.
12.3 Pharmacokinetics
Absorption
Absorption of orally administered T4 from the gastrointestinal (GI) tract ranges from 40% to 80%. The majority of the levothyroxine dose is absorbed from the jejunum and upper ileum. The relative bioavailability of TIROSINT-SOL compared to TIROSINT capsules, is approximately 98%. T4 absorption is increased by fasting, and decreased in malabsorption syndromes and by certain foods such as soybeans. Dietary fiber decreases the bioavailability of T4. Absorption may also decrease with age. In addition, many drugs and foods affect T4 absorption [see Drug Interactions (7)].
Distribution
Circulating thyroid hormones are greater than 99% bound to plasma proteins, including thyroxine-binding globulin (TBG), thyroxine-binding prealbumin (TBPA), and thyroxine-binding albumin (TBA), whose capacities and affinities vary for each hormone. The higher affinity of both TBG and TBPA for T4 partially explains the higher serum levels, slower metabolic clearance, and longer half-life of T4 compared to T3. Protein-bound thyroid hormones exist in reverse equilibrium with small amounts of free hormone. Only unbound hormone is metabolically active. Many drugs and physiologic conditions affect the binding of thyroid hormones to serum proteins [see Drug Interactions (7)]. Thyroid hormones do not readily cross the placental barrier [see Use in Specific Populations (8.1)].
Elimination
Metabolism
T4 is slowly eliminated (see Table 6). The major pathway of thyroid hormone metabolism is through sequential deiodination. Approximately 80% of circulating T3 is derived from peripheral T4 by monodeiodination. The liver is the major site of degradation for both T4 and T3, with T4 deiodination also occurring at a number of additional sites, including the kidney and other tissues. Approximately 80% of the daily dose of T4 is deiodinated to yield equal amounts of T3 and reverse T3 (rT3). T3 and rT3 are further deiodinated to diiodothyronine. Thyroid hormones are also metabolized via conjugation with glucuronides and sulfates and excreted directly into the bile and gut where they undergo enterohepatic recirculation.
Excretion
Thyroid hormones are primarily eliminated by the kidneys. A portion of the conjugated hormone reaches the colon unchanged and is eliminated in the feces. Approximately 20% of T4 is eliminated in the stool. Urinary excretion of T4 decreases with age.
Table 6: Pharmacokinetic Parameters of Thyroid Hormones in Euthyroid Patients Hormone Ratio in Thyroglobulin Biologic Potency Half-Life
(Days)Protein Binding
(%)*- * Includes TBG, TBPA and TBA.
- † 3 – 4 days in hyperthyroidism, 9 to 10 days in hypothyroidism.
Levothyroxine (T4) 10 – 20 1 6 – 7† 99.96 Liothyronine (T3) 1 4 ≤ 2 99.5 - 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TIROSINT-SOL (levothyroxine sodium) oral solution is a clear, colorless to slightly yellow solution supplied in a 1 mL white, non-transparent, unit-dose ampule. The dosage strength is identified on the box and the pouch, and is associated with a distinct color. Each ampule bears a colored label with the dosage strength and the product name (TIROSINT-SOL).
Table 7: TIROSINT-SOL Packaging Description Strength
(mcg/mL)Color* Box NDC
(30 Unit-Dose Ampules)Pouch NDC
(5 Unit-Dose Ampules)- * Shown on box, pouch and ampule.
13 Green 71858-0105-5 71858-0105-4 25 Orange 71858-0110-5 71858-0110-4 50 White 71858-0115-5 71858-0115-4 75 Purple 71858-0120-5 71858-0120-4 88 Olive 71858-0125-5 71858-0125-4 100 Yellow 71858-0130-5 71858-0130-4 112 Rose 71858-0135-5 71858-0135-4 125 Brown 71858-0140-5 71858-0140-4 137 Turquoise 71858-0145-5 71858-0145-4 150 Blue 71858-0150-5 71858-0150-4 175 Lilac 71858-0155-5 71858-0155-4 200 Pink 71858-0160-5 71858-0160-4 16.2 Storage and Handling
Store TIROSINT-SOL in the original container (closed pouch) at 25°C (77°F); excursions permitted to 15°-30°C (59-86°F) [See USP Controlled Room Temperature].
Use TIROSINT-SOL oral solution within 15 days after opening the pouch. Keep the ampules in the pouch until ready to use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Dosing and Administration
- Instruct patients to take TIROSINT-SOL only as directed by their healthcare provider.
- Instruct patients to take TIROSINT-SOL one-half to one hour before breakfast.
- Instruct patients about the TIROSINT-SOL dosing instructions [see Dosage and Administration (2.1)].
- Inform patients that agents such as iron and calcium supplements and antacids can decrease the absorption of levothyroxine. Instruct patients not to take TIROSINT-SOL within 4 hours of these agents.
- Instruct patients to notify their healthcare provider should they become pregnant or are thinking of becoming pregnant while taking TIROSINT-SOL.
Important Information
- Inform patients that it may take several weeks before they notice an improvement in symptoms.
- Inform patients that the levothyroxine in TIROSINT-SOL is intended to replace a hormone that is normally produced by the thyroid gland. Generally, replacement therapy is to be taken for life.
- Inform patients that TIROSINT-SOL should not be used as a primary or adjunctive therapy in a weight control program.
- Instruct patients to notify their healthcare provider if they are taking any other medications, including prescription and over-the-counter preparations [see Drug Interactions (7)].
- Instruct patients to notify their healthcare provider of any other medical conditions, particularly heart disease, diabetes, clotting disorders, and adrenal or pituitary gland problems, as the dose of medications used to control these other conditions may need to be adjusted while taking TIROSINT-SOL. If they have diabetes, instruct patients to monitor their blood and/or urinary glucose levels as directed by their physician and immediately report any changes to their physician. If patients are taking anticoagulants, their clotting status should be checked frequently.
- Instruct patients to notify their physician or dentist that they are taking TIROSINT-SOL prior to any surgery.
Adverse Reactions
- Instruct patients to notify their healthcare provider if they experience any of the following symptoms: rapid or irregular heartbeat, chest pain, shortness of breath, leg cramps, headache, nervousness, irritability, sleeplessness, tremors, change in appetite, weight loss, vomiting, diarrhea, excessive sweating, heat intolerance, fever, changes in menstrual periods, hives or skin rash, or any other unusual medical event.
- Inform patients that partial hair loss may occur rarely during the first few months of TIROSINT-SOL therapy, but this is usually temporary.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
TIROSINT®-SOL [tee-row-sent-sōl]
(levothyroxine sodium)
oral solutionWhat is the most important information I should know about TIROSINT-SOL?
Do not use TIROSINT-SOL to treat weight problems or weight loss.
What is TIROSINT-SOL?
TIROSINT-SOL is a prescription medicine that contains a hormone called levothyroxine which is normally produced by the thyroid gland. TIROSINT-SOL is used:
- to replace or give extra levothyroxine in people whose thyroid does not produce enough of this hormone; or
- with surgery and radiodine therapy to manage a type of thyroid cancer called thyroid-dependent well-differentiated thyroid cancer.
TIROSINT-SOL should not be used to treat people who are recovering from swelling of the thyroid gland (thyroiditis) and whose bodies do not produce enough levothyroxine for a short time.
Do not take TIROSINT-SOL:
- if you are allergic to glycerol the inactive ingredient in TIROSINT-SOL; or
- if your adrenal glands are not working well and you have not been treated for this problem.
Before you take TIROSINT-SOL, tell your doctor about all of your medical conditions, including if you:
- have or have had heart problems
- have or have had thyroid nodules
- have adrenal or pituitary gland problems
- have any food or drug allergies
- have low red blood cell count (anemia)
- have diabetes
- have weak bones (osteoporosis)
- have or had a history of blood clotting problems
- have recently received radiation therapy with iodine (such as I-131)
- are pregnant or plan to become pregnant. Your doctor may need to change your TIROSINT-SOL dose while you are pregnant.
- are breastfeeding. TIROSINT-SOL can pass into your milk. Talk to your doctor about the best way to feed your baby if you take TIROSINT-SOL.
Tell your doctor about all the medicines you are taking including prescription and over-the-counter medicines, vitamins and herbal supplements. TIROSINT-SOL may affect the way other medicines work, and other medicines may affect how TIROSINT-SOL works so your doctor may have to adjust the amount of medicines you take. You can ask your doctor or pharmacist for a list of medicines that interact with TIROSINT-SOL.
How should I take TIROSINT-SOL?
- TIROSINT-SOL is for oral use only. Do not inhale, inject, or place TIROSINT-SOL in the eyes.
- See the detailed "Instructions for Use" that come with TIROSINT-SOL for information on the right way to take your dose of TIROSINT-SOL oral solution.
- Take TIROSINT-SOL exactly as your doctor tells you to take it.
- Your doctor will tell you how much TIROSINT-SOL to take each day.
- Your doctor may change your dose, if needed.
- Take your dose of TIROSINT-SOL 1 time each day 30 minutes to 1 hour before breakfast on an empty stomach.
- Certain medicines can interfere with how TIROSINT-SOL is absorbed by your body. Take TIROSINT-SOL:
- at least 4 hours before or after you take medicines that contain calcium carbonate or iron (ferrous sulfate); and
- at least 4 hours before you take medicines that contain bile acid sequestrants or ion exchange resins.
Know the medicines you take. Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
- Certain foods including soybean flour, cotton seed meal, walnuts, and dietary fiber can affect your treatment and dose of TIROSINT-SOL. Talk to your doctor if you eat or drink these foods.
- Do not remove TIROSINT–SOL ampules from the sealed aluminum foil pouch until you are ready to use them.
- Use all 5 TIROSINT-SOL ampules within 15 days after opening the aluminum foil pouch.
- Your doctor should do certain blood tests while you are taking TIROSINT-SOL and may change your daily dose of TIROSINT-SOL as needed. Keep taking TIROSINT-SOL unless your doctor tells you to stop or to change your dose.
It may take weeks before you notice your symptoms getting better. Keep using this medicine even if you feel well. If you take too much TIROSINT-SOL, call your doctor, your Poison Control Center at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What are the possible side effects of TIROSINT-SOL?
TIROSINT-SOL may cause serious side effects, including:
- heart problems. You may experience an increased heart rate, chest pain and irregular heartbeat. Your risk of developing heart problems may be greater if you are elderly, you have heart problems, or you take too much TIROSINT-SOL. Your doctor may reduce your dose or stop treatment with TIROSINT-SOL for a while if you develop heart problems.
- worsening diabetic control. If you are diabetic, it may be harder to control your blood sugar levels causing hyperglycemia while taking TIROSINT-SOL. Check your blood sugar levels closely after starting, changing, or stopping treatment with TIROSINT-SOL. Your doctor may have to change your diabetes treatment plan.
- weak or brittle bones. Your risk of developing weak or brittle bones may be greater if you are post-menopausal or you take too much TIROSINT-SOL.
The most common side effects of TIROSINT-SOL include:
- fast or irregular heartbeat
- chest pain
- shortness of breath
- leg cramps
- headache
- nervousness
- hives or skin rash
- irritability
- sleep problems (insomnia)
- tremors
- muscle weakness
- change in appetite
- weight loss
- vomiting
- diarrhea
- sweating a lot
- heat intolerance
- fever
- changes in menstrual period
Other side effects may include partial hair loss during the first months of treatment with TIROSINT-SOL. This usually lasts a short period of time (temporary).
These are not all the possible side effects of TIROSINT-SOL. Call your doctor for medical advice about side effects. You may report side effects to IBSA Pharma Inc. at 1-800-587-3513 or FDA at 1-800-FDA-1088.
How should I store TIROSINT-SOL?
- Store TIROSINT-SOL at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TIROSINT-SOL in the original closed pouch until you are ready to use it.
- Do not store the diluted or mixed TIROSINT-SOL solution.
Keep TIROSINT-SOL and all medicines out of the reach of children.
General information about the safe and effective use of TIROSINT-SOL
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TIROSINT-SOL for a condition for which it was not prescribed. Do not give TIROSINT-SOL to other people, even if they have the same symptoms as you. It may harm them. You can ask your pharmacist or doctor for information about TIROSINT-SOL that is written for health professionals.
What are the ingredients in TIROSINT- SOL oral solution?
Active ingredient: levothyroxine sodium
Inactive ingredients: glycerol, and water
Manufactured by: IBSA Institut Biochimique SA, 6915 Pambio-Noranco, Switzerland
Marketed and distributed by: IBSA Pharma Inc, Parsippany, NJ 07054 USA
For more information, go to www.tirosint-sol.com or call 1-800-587-3513.This Patient Information has been approved by the U.S. Food and Drug Administration
Issued: June 2018 -
INSTRUCTIONS FOR USE
Instructions for Use
TIROSINT®-SOL (tee-row-sent-sōl)
(levothyroxine sodium)
oral solutionRead this Instructions for Use before you start taking TIROSINT-SOL and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information:
- TIROSINT-SOL is for oral use only. Do not inhale, inject, or place TIROSINT-SOL in the eyes.
- TIROSINT-SOL can be taken:
- by diluting or mixing in water first or
- by directly squeezing into the mouth or onto a spoon.
- Do not dilute or mix TIROSINT-SOL with any liquid other than water.
- Open the ampule and prepare the solution right before you take TIROSINT-SOL.
- After you have diluted or mixed TIROSINT-SOL it must be taken or thrown away.
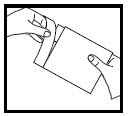
Step 1. Open the aluminum pouch by tearing the edge along the dotted line (See Figure A).
Figure A
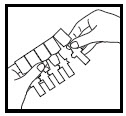
Step 2. Remove 1 ampule from the strip to be used right away (See Figure B). Return the unused ampules back into the pouch before storing.
Figure B
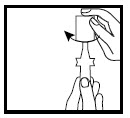
Step 3. Hold the TIROSINT-SOL ampule upright (cap on top) between the first finger and thumb without squeezing the ampule. Open the ampule by twisting off the top (See Figure C).
Figure C
Step 4.
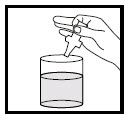
Taking TIROSINT-SOL by diluting or mixing:
- Turn the ampule upside down.
- Squeeze the middle, softer part of the ampule between the first finger and thumb slowly to release the liquid medicine into a glass or cup containing water, then release the pressure and wait a few seconds (See Figure D).
- Keeping the ampule upside down, repeat this step for a minimum of 5 times, until no more liquid medicine comes out of the ampule.
- Stir the solution.
- Drink all the liquid medicine right away.
- Rinse the glass or cup with more water and drink to make sure all the medicine has been taken.
Figure D
Taking TIROSINT-SOL directly into the mouth or by spoon:
- Turn the ampule upside down.
- Squeeze the middle, softer part of the ampule between the first finger and thumb slowly to release the liquid medicine into the mouth or onto a spoon, then release the pressure and wait a few seconds (See Figure E).
- Keeping the ampule upside down, repeat this step for a minimum of 5 times, until no more liquid medicine comes out of the ampule.
Figure E
Step 5. Throw away (discard) the empty ampule.
How should I store TIROSINT-SOL?
- Store TIROSINT-SOL at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TIROSINT-SOL in the original closed pouch until you are ready to use it.
- Do not store the diluted or mixed TIROSINT-SOL solution.
Keep TIROSINT-SOL and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufacturer: IBSA Institut Biochimique SA, 6915 Pambio-Noranco, Switzerland
Marketed and distributed by: IBSA Pharma Inc., Parsippany, NJ 07054 USAIssued: June 2018
-
PRINCIPAL DISPLAY PANEL - 13 microgram/mL Ampule Pouch Carton
NDC: 71858-0105-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution13 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
PRINCIPAL DISPLAY PANEL - 25 microgram/mL Ampule Pouch Carton
NDC: 71858-0110-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution25 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
PRINCIPAL DISPLAY PANEL - 50 microgram/mL Ampule Pouch Carton
NDC: 71858-0115-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution50 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
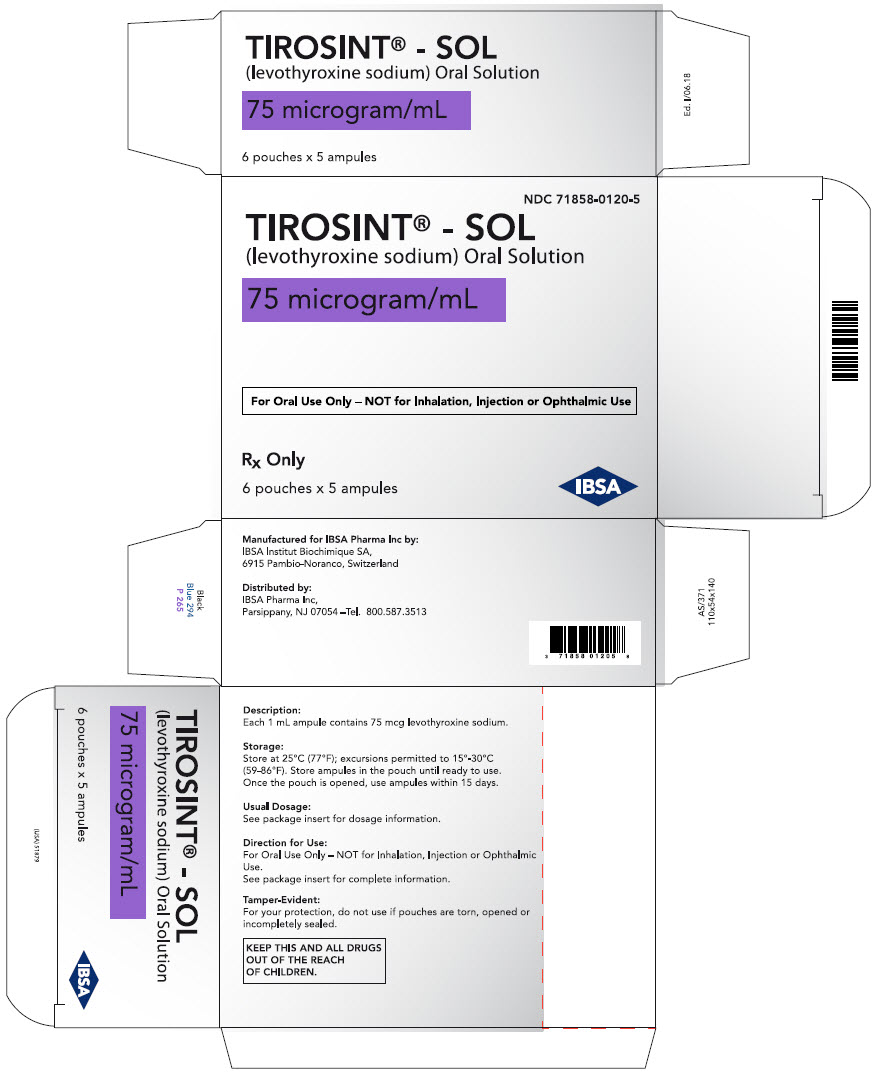
PRINCIPAL DISPLAY PANEL - 75 microgram/mL Ampule Pouch Carton
NDC: 71858-0120-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution75 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
PRINCIPAL DISPLAY PANEL - 88 microgram/mL Ampule Pouch Carton
NDC: 71858-0125-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution88 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
PRINCIPAL DISPLAY PANEL - 100 microgram/mL Ampule Pouch Carton
NDC: 71858-0130-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution100 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
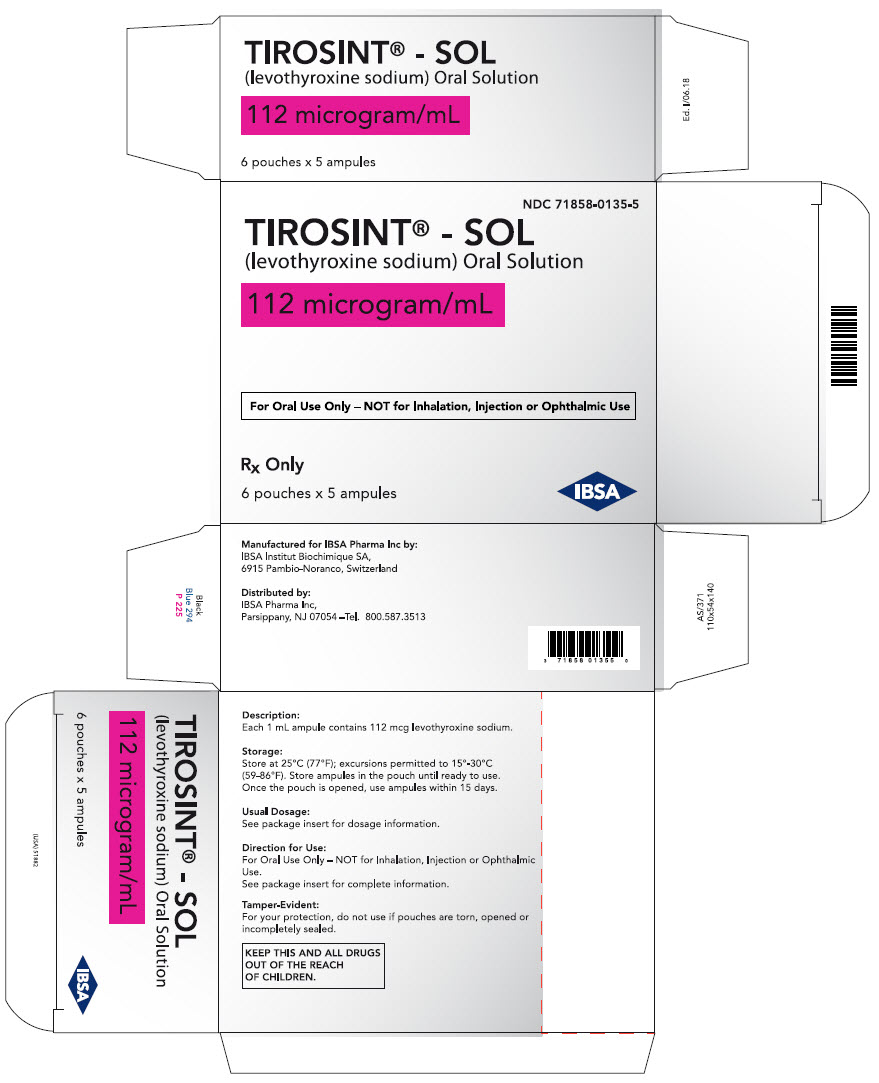
PRINCIPAL DISPLAY PANEL - 112 microgram/mL Ampule Pouch Carton
NDC: 71858-0135-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution112 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
PRINCIPAL DISPLAY PANEL - 125 microgram/mL Ampule Pouch Carton
NDC: 71858-0140-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution125 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
PRINCIPAL DISPLAY PANEL - 137 microgram/mL Ampule Pouch Carton
NDC: 71858-0145-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution137 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
PRINCIPAL DISPLAY PANEL - 150 microgram/mL Ampule Pouch Carton
NDC: 71858-0150-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution150 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA

-
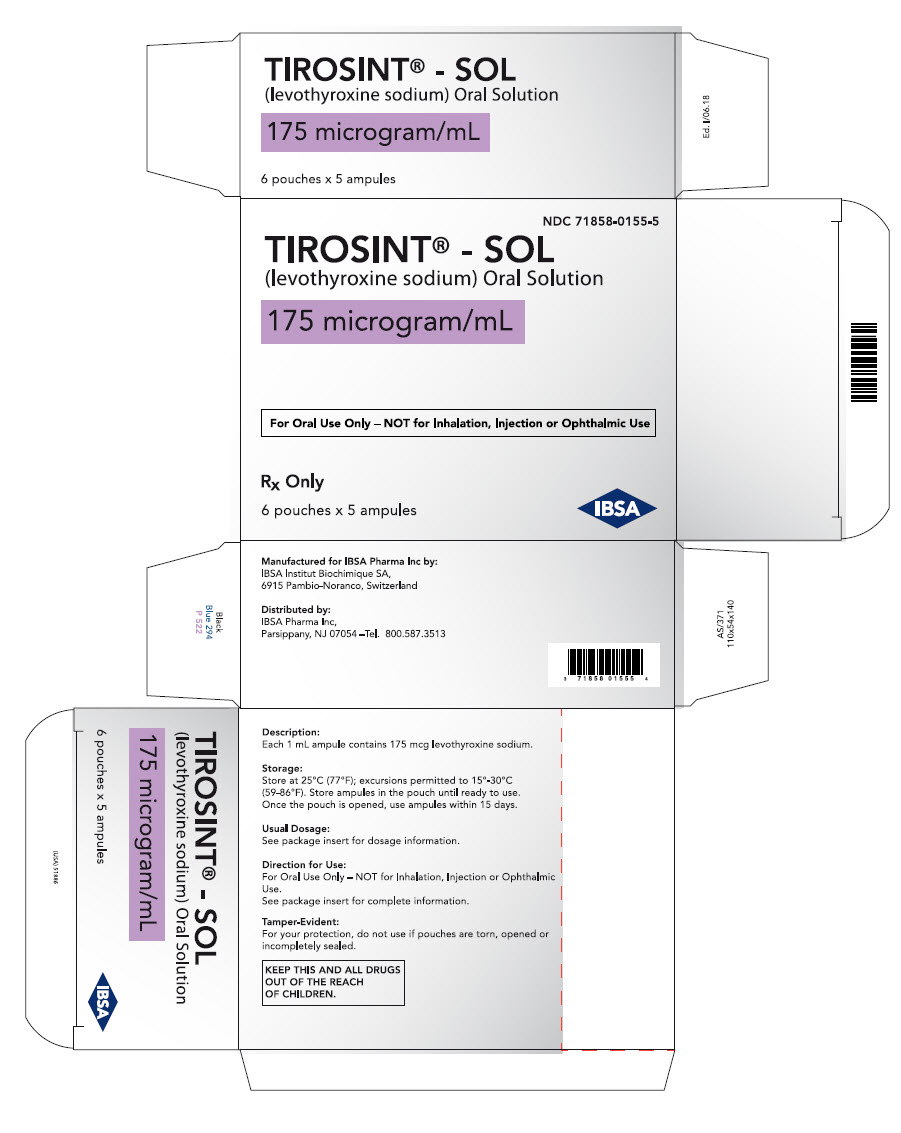
PRINCIPAL DISPLAY PANEL - 175 microgram/mL Ampule Pouch Carton
NDC: 71858-0155-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution175 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
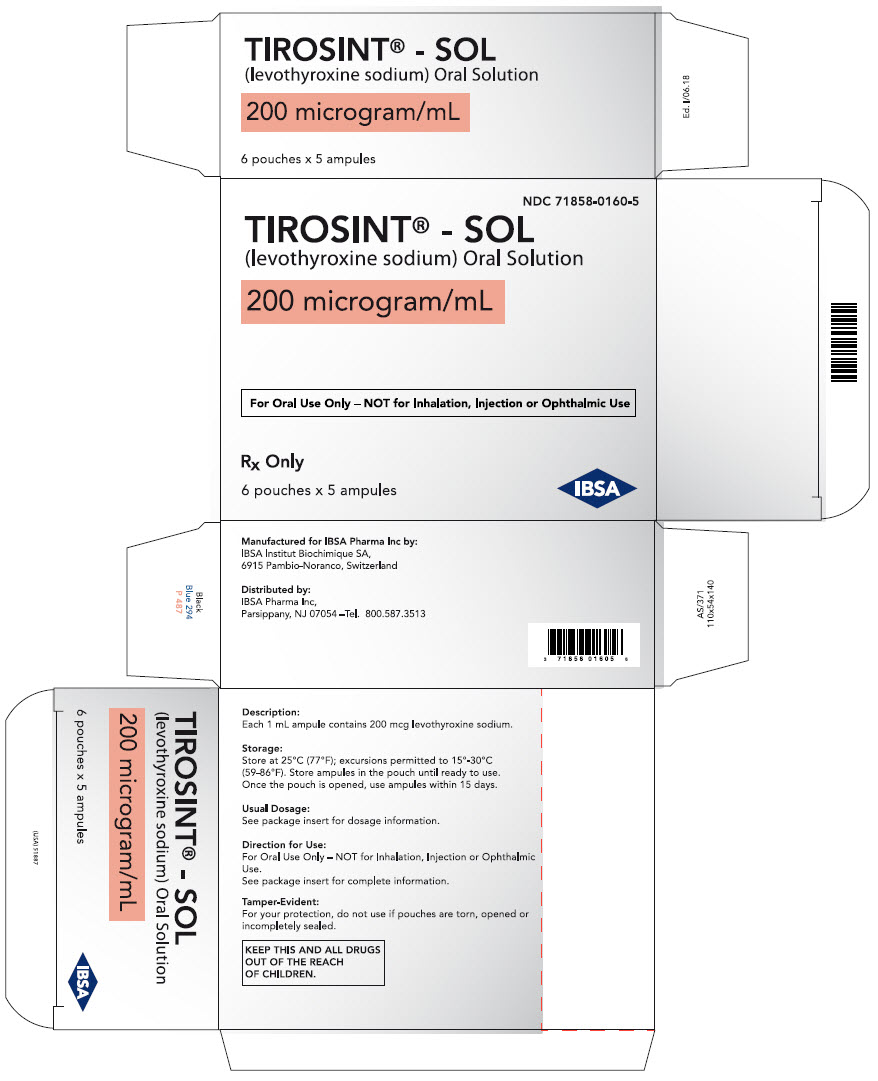
PRINCIPAL DISPLAY PANEL - 200 microgram/mL Ampule Pouch Carton
NDC: 71858-0160-5
TIROSINT® - SOL
(levothyroxine sodium) Oral Solution200 microgram/mL
For Oral Use Only – NOT for Inhalation, Injection or Ophthalmic Use
Rx Only
6 pouches x 5 ampules
IBSA
-
INGREDIENTS AND APPEARANCE
TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 13 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0105-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0105-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0105-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0105-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 25 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0110-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0110-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0110-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0110-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 50 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0115-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0115-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0115-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0115-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 75 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0120-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0120-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0120-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0120-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 88 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0125-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0125-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0125-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0125-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0130 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0130-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0130-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0130-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0130-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0135 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 112 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0135-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0135-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0135-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0135-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 125 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0140-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0140-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0140-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0140-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0145 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 137 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0145-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0145-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0145-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0145-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 150 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0150-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0150-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0150-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0150-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0155 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 175 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0155-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0155-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0155-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0155-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 TIROSINT SOL
levothyroxine sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71858-0160 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levothyroxine Sodium (UNII: 9J765S329G) (Levothyroxine - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 200 ug in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71858-0160-5 6 in 1 CARTON 03/01/2019 1 NDC: 71858-0160-4 5 in 1 POUCH 1 1 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 71858-0160-1 2 in 1 CARTON 03/01/2019 2 NDC: 71858-0160-4 5 in 1 POUCH 2 1 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206977 03/01/2019 Labeler - IBSA Pharma Inc. (081215551) Registrant - IBSA Pambio Noranco Complex (485251214)
Trademark Results [Tirosint]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TIROSINT 79011405 3154257 Live/Registered |
IBSA Institut Biochimique S.A. 2005-04-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.