TAFLUPROST solution/ drops
Tafluprost by
Drug Labeling and Warnings
Tafluprost by is a Prescription medication manufactured, distributed, or labeled by Ingenus Pharmaceuticals, LLC, Kaysersberg Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TAFLUPROST OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for TAFLUPROST OPHTHALMIC SOLUTION.
TAFLUPROST ophthalmic solution
Initial U.S. Approval: 2012INDICATIONS AND USAGE
- Tafluprost ophthalmic solution, 0.0015% is a prostaglandin analog indicated for reducing elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. (1)
DOSAGE AND ADMINISTRATION
- One drop in the affected eye(s) once daily in the evening. (2)
DOSAGE FORMS AND STRENGTHS
- Ophthalmic solution containing tafluprost 0.015 mg/mL. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- Most common ocular adverse reaction is conjunctival hyperemia (range 4% – 20%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Use in pediatric patients is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
5.2 Eyelash Changes
5.3 Intraocular Inflammation
5.4 Macular Edema
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Nightly Application
17.2 Handling the Single-Dose Container
17.3 Potential for Pigmentation
17.4 Potential for Eyelash Changes
17.5 When to Seek Physician Advice
17.6 Use with Other Ophthalmic Drugs
17.7 Storage Information
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dose is one drop of tafluprost ophthalmic solution in the conjunctival sac of the affected eye(s) once daily in the evening.
The dose should not exceed once daily since it has been shown that more frequent administration of prostaglandin analogs may lessen the intraocular pressure lowering effect.
Reduction of the intraocular pressure starts approximately 2 to 4 hours after the first administration with the maximum effect reached after 12 hours.
Tafluprost ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is being used, each one should be administered at least 5 minutes apart.
The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Tafluprost ophthalmic solution has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as tafluprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of tafluprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with tafluprost ophthalmic solution can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly. [See Patient Counseling Information (17.3)].
5.2 Eyelash Changes
Tafluprost ophthalmic solution may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, color, thickness, shape and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
Tafluprost ophthalmic solution should be used with caution in patients with active intraocular inflammation (e.g., iritis/uveitis) because the inflammation may be exacerbated.
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with prostaglandin F2α analogs. Tafluprost ophthalmic solution should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Preservative-containing or preservative-free tafluprost 0.0015% was evaluated in 905 patients in five controlled clinical studies of up to 24-months duration. The most common adverse reaction observed in patients treated with tafluprost was conjunctival hyperemia which was reported in a range of 4% to 20% of patients. Approximately 1% of patients discontinued therapy due to ocular adverse reactions.
Ocular adverse reactions reported at an incidence of ≥ 2% in these clinical studies included ocular stinging/irritation (7%), ocular pruritus including allergic conjunctivitis (5%), cataract (3%), dry eye (3%), ocular pain (3%), eyelash darkening (2%), growth of eyelashes (2%) and vision blurred (2%).
Nonocular adverse reactions reported at an incidence of 2% to 6% in these clinical studies in patients treated with tafluprost 0.0015% were headache (6%), common cold (4%), cough (3%) and urinary tract infection (2%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of tafluprost. Because postapproval adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Respiratory disorders: exacerbation of asthma, dyspnea
Eye disorders: iritis/uveitis
In postmarketing use with prostaglandin analogs, periorbital and lid changes including deepening of the eyelid sulcus have been observed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: In embryo-fetal development studies in rats and rabbits, tafluprost administered intravenously was teratogenic. Tafluprost caused increases in post-implantation losses in rats and rabbits and reductions in fetal body weights in rats. Tafluprost also increased the incidence of vertebral skeletal abnormalities in rats and the incidence of skull, brain and spine malformations in rabbits. In rats, there were no adverse effects on embryo-fetal development at a dose of 3 mcg/kg/day corresponding to maternal plasma levels of tafluprost acid that were 343 times the maximum clinical exposure based on Cmax. In rabbits, effects were seen at a tafluprost dose of 0.03 mcg/kg/day corresponding to maternal plasma levels of tafluprost acid during organogenesis that were approximately 5 times higher than the clinical exposure based on Cmax. At the no-effect dose in rabbits (0.01 mcg/kg/day), maternal plasma levels of tafluprost acid were below the lower level of quantification (20 pg/mL).
In a pre- and postnatal development study in rats, increased mortality of newborns, decreased body weights and delayed pinna unfolding were observed in offsprings. The no observed adverse effect level was at a tafluprost intravenous dose of 0.3 mcg/kg/day which is greater than 3 times the maximum recommended clinical dose based on body surface area comparison.
There are no adequate and well-controlled studies in pregnant woman. Although animal reproduction studies are not always predictive of human response, tafluprost ophthalmic solution should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Women of childbearing age/potential should have adequate contraceptive measures in place.
8.3 Nursing Mothers
A study in lactating rats demonstrated that radio-labeled tafluprost and/or its metabolites were excreted in milk. It is not known whether this drug or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tafluprost ophthalmic solution is administered to a nursing woman.
-
11 DESCRIPTION
Tafluprost is a fluorinated analog of prostaglandin F2α. The chemical name for tafluprost is Propan-2-yl (5Z)-7-{(1R,2R,3R,5S)-2-[(1E)-3,3 difluoro-4-phenoxybut-1-enyl]-3,5-dihydroxycyclopentyl}hept-5-enoate. The molecular formula of tafluprost is C25H34F2O5 and its molecular weight is 452.5.
Its structural formula is:
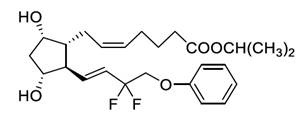
Tafluprost is a colorless to yellowish hygroscopic oil that is very soluble in acetone, acetonitrile, dichloromethane, diethylether, ethanol, methanol and tert-butyl(methyl)ether and practically insoluble in n-heptane and water.
Tafluprost ophthalmic solution, 0.0015% is supplied as a sterile solution of tafluprost with a pH range of 5.5 to 6.7 and an Osmolality range of 260 to 300 mOsmol/kg.
Tafluprost ophthalmic solution contains Active: tafluprost 0.015 mg/mL; Inactives: glycerin, monobasic sodium phosphate dihydrate, edetate disodium dihydrate, polysorbate 80, hydrochloric acid and/or sodium hydroxide (to adjust pH) and Water for Injection.
Tafluprost ophthalmic solution does not contain a preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tafluprost acid, a prostaglandin analog is a selective FP prostanoid receptor agonist which is believed to reduce intraocular pressure by increasing uveoscleral outflow. The exact mechanism of action is unknown at this time.
12.3 Pharmacokinetics
Following instillation, tafluprost is absorbed through the cornea and is hydrolyzed to the biologically active acid metabolite, tafluprost acid. Following instillation of one drop of the 0.0015% solution once daily into each eye of healthy volunteers, the plasma concentrations of tafluprost acid peaked at a median time of 10 minutes on both Days 1 and 8. The mean plasma Cmax of tafluprost acid were 26 pg/mL and 27 pg/mL on Day 1, and Day 8, respectively. The mean plasma AUC estimates of tafluprost acid were 394 pg*min/mL and 432 pg*min/mL on Day 1 and 8, respectively.
Metabolism
Tafluprost, an ester prodrug, is hydrolyzed to its biologically active acid metabolite in the eye. The acid metabolite is further metabolized via fatty acid β-oxidation and phase II conjugation.
Elimination
Mean plasma tafluprost acid concentrations were below the limit of quantification of the bioanalytical assay (10 pg/mL) at 30 minutes following topical ocular administration of tafluprost 0.0015% ophthalmic solution.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Tafluprost was not carcinogenic when administered subcutaneously daily for 24 months at doses up to 30 mcg/kg/day in rats and for 18 months at doses up to 100 mcg/kg/day in mice (over 1600 and 1300 times, respectively, the maximum clinical exposure based on plasma AUC).
Tafluprost was not mutagenic or clastogenic in a battery of genetic toxicology studies, including an in vitro microbial mutagenesis assay, an in vitro chromosomal aberration assay in Chinese hamster lung cells, and an in vivo mouse micronucleus assay in bone marrow.
In rats, no adverse effects on mating performance or fertility were observed with intravenous dosing of tafluprost at a dose of 100 mcg/kg/day (over 14000 times the maximum clinical exposure based on plasma Cmax or over 3600 times based on plasma AUC).
-
14 CLINICAL STUDIES
In clinical studies up to 24 months in duration, patients with open-angle glaucoma or ocular hypertension and baseline pressure of 23 to 26 mm Hg who were treated with tafluprost ophthalmic solution dosed once daily in the evening demonstrated reductions in intraocular pressure at 3 and 6 months of 6 to 8 mmHg and 5 to 8 mmHg, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tafluprost ophthalmic solution, 0.0015% is supplied as a sterile solution in translucent low density polyethylene single-dose containers packaged in foil pouches (5 single-dose containers per pouch). Each single-dose container has 0.3 mL solution corresponding to 0.0045 mg tafluprost.
NDC: 50742-339-30; Unit-of-Use Carton of 30.
Storage:
Store refrigerated at 2º to 8°C (36º to 46°F). During shipment tafluprost ophthalmic solution may be maintained at temperatures up to 40ºC (104ºF) for a period not exceeding 2 days. Mail-order prescriptions received after two days of the dispensing date noted on the prescribing label should not be used. Store in the original pouch. After the pouch is opened, the single-dose containers may be stored in the opened foil pouch for up to 30 days at room temperature 20º to 25°C (68º to 77°F). Protect from moisture. Write down the date you open the foil pouch in the space provided on the pouch. Discard any unused containers 30 days after first opening the pouch.
-
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information).
17.1 Nightly Application
Advise patients to not exceed once daily dosing since more frequent administration may decrease the intraocular pressure lowering effect of tafluprost ophthalmic solution.
17.2 Handling the Single-Dose Container
Advise patients that tafluprost ophthalmic solution is a sterile solution that does not contain a preservative. The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
17.3 Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Also inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of tafluprost ophthalmic solution.
17.4 Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with tafluprost ophthalmic solution. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
17.5 When to Seek Physician Advice
Advise patients that if they develop a new ocular condition (e.g., trauma or infection), experience a sudden decrease in visual acuity, have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of tafluprost ophthalmic solution.
17.6 Use with Other Ophthalmic Drugs
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes between applications.
17.7 Storage Information
Instruct patients on proper storage of cartons, unopened foil pouches, and opened foil pouches [see How Supplied/Storage and Handling (16)]. Recommended storage for cartons and unopened foil pouches is to store refrigerated at 2º to 8°C (36º to 46°F). After the pouch is opened, the single-dose containers may be stored in the opened foil pouch for up to 28 days at room temperature 20º to 25°C (68º to 77°F). Protect from moisture.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811-7193
Made in France
Rx Only

-
SPL PATIENT PACKAGE INSERT
Patient Information
Tafluprost Ophthalmic Solution, 0.0015%
(ta-floo-prost)
Read this Patient Information before you start using tafluprost ophthalmic solution and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is tafluprost ophthalmic solution?
Tafluprost ophthalmic solution is a prescription sterile eye drop solution. Tafluprost ophthalmic solution is used to lower the pressure in the eye (intraocular pressure) in people with open-angle glaucoma or ocular hypertension when their eye pressure is too high. Tafluprost ophthalmic solution belongs to a group of medicines called prostaglandin analogs.
Tafluprost ophthalmic solution is not for use in children.
What should I tell my doctor before using tafluprost ophthalmic solution?
Before you use tafluprost ophthalmic solution, tell your doctor if you:
● have or have had eye problems including any surgery on your eye or eyes
● are using any other eye medicines
● have any other medical problems
● are pregnant or plan to become pregnant. It is not known if tafluprost ophthalmic solution will harm your unborn baby. You should use an effective method of birth control while you use tafluprost ophthalmic solution. If you become pregnant while using tafluprost ophthalmic solution talk to your doctor right away.
● are breastfeeding or plan to breastfeed. It is not known if tafluprost ophthalmic solution passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use tafluprost ophthalmic solution.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take tafluprost ophthalmic solution?
Read the Instructions for Use at the end of this Patient Information leaflet for additional instructions about the right way to use tafluprost ophthalmic solution.
● Use 1 drop of tafluprost ophthalmic solution in your eye (or eyes) each evening. Talk to your doctor or pharmacist if you are not sure how to use tafluprost ophthalmic solution.
● Your tafluprost ophthalmic solution may not work as well if you use it more than 1 time each evening.
● If you use other medicines in your eye, wait at least 5 minutes between using tafluprost ophthalmic solution and your other eye medicines.
● Use your tafluprost ophthalmic solution right away after opening. Each tafluprost ophthalmic solution single-dose container is sterile and is to be used 1 time then thrown away. Do not save any tafluprost ophthalmic solution that may be left over after you use your medicine. Using tafluprost ophthalmic solution that is not sterile may cause other eye problems.
What are the possible side effects of tafluprost ophthalmic solution?
Tafluprost ophthalmic solution may cause serious side effects including:
● changes in the color of your eye (iris). Your iris may become more brown in color while using tafluprost ophthalmic solution. This color change may not go away when you stop using tafluprost ophthalmic solution. If tafluprost ophthalmic solution is used in 1 eye only, the color of that eye may always be a different color from the color of your other eye.
● darkening of the color of the skin around your eye (eyelid). These skin changes usually go away when you stop using tafluprost ophthalmic solution.
● increasing the length, thickness, color, or number of your eyelashes. These eyelash changes usually go away when you stop using tafluprost ophthalmic solution.
● hair growth on your eyelids. This hair growth usually goes away when you stop using tafluprost ophthalmic solution.
The most common side effects of tafluprost ophthalmic solution include:
● redness, stinging or itching of your eye
● cataract formation
● dry eye
● eye pain
● blurred vision
● headache
● common cold
● cough
● urinary tract infection
Tell your doctor if you have any new eye problems while using tafluprost ophthalmic solution including:
● an eye injury
● an eye infection
● a sudden loss of vision
● eye surgery
● swelling and redness of and around your eye (conjunctivitis)
● problems with your eyelids
Additionally, the following side effects have been reported in general use:
● worsening of asthma
● shortness of breath
Tell your doctor if you have any other side effects that bother you.
These are not all the possible side effects of tafluprost ophthalmic solution. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store tafluprost ophthalmic solution?
Important Information for Mail-Order Patients:
Do not use if prescription is not received within two days of dispensing date.
Keep the foil pouches and tafluprost ophthalmic solution single-dose containers dry. Before opening the foil pouches:
● Store the unopened foil pouches in a refrigerator between 36°F to 46°F (2°C to 8°C).
● Do not open the pouch containing tafluprost ophthalmic solutionuntil you are ready to use the eye drops.
After opening the foil pouch:
● Store the opened foil pouch at room temperature, between 68°F to 77°F (20°C to 25°C), for up to 30 days.
● Throw away all unused tafluprost ophthalmic solution single-dose containers in the opened foil pouch after 30 days.
● Keep the tafluprost ophthalmic solution single-dose containers in their original foil pouch.
● After opening the foil pouch, refrigeration is not required.
Keep tafluprost ophthalmic solutionand all medicines out of the reach of children.
General information about the safe and effective use of tafluprost ophthalmic solution.
Do not use tafluprost ophthalmic solutionfor a condition for which it was not prescribed. Do not give tafluprost ophthalmic solutionto other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about tafluprost ophthalmic solution. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about tafluprost ophthalmic solution that is written for health professionals.
What are the ingredients in tafluprost ophthalmic solution?
Active ingredients: tafluprost
Inactive ingredients: glycerin, monobasic sodium phosphate dihydrate, edetate disodium dihydrate, and polysorbate 80, hydrochloric acid and/or sodium hydroxide, and water for Injection.
-
INSTRUCTIONS FOR USE SECTION
Instructions for Use
Read these Instructions for Use before using your tafluprost ophthalmic solutionand each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
Important:
● Tafluprost ophthalmic solutionis for the eye only. Do not swallow tafluprost ophthalmic solution.
● Tafluprost ophthalmic solution single-dose containers are packaged in a foil pouch.
● Do not use the tafluprost ophthalmic solution single-dose containers if the foil pouch is opened.
● Write down the date you open the foil pouch in the space provided on the pouch.
Every time you use tafluprost ophthalmic solution:
Step 1. Wash your hands.
Step 2. Take the strip of single-dose containers from the foil pouch.
Step 3. Pull off one single-dose container from the strip.
Step 4. Put the remaining strip of single-dose containers back in the foil pouch and fold the edge to close the pouch.
Step 5. Hold the single-dose container upright. Make sure that your tafluprost ophthalmic solution medicine is in the bottom part of the single-dose container.
See Figure A.
Step 6. Open the single-dose container by twisting off the tab.
See Figure B .
Step 7. Tilt your head backwards. If you are unable to tilt your head, lie down.
Step 8 . Place the tip of the single-dose container close to your eye. Be careful not to touch your eye with the tip of the single-dose container
See Figure C .
Step 9 . Pull your lower eyelid downwards and look up.
Step 10. Gently squeeze the container and let 1 drop of tafluprost ophthalmic solution fall into the space between your lower eyelid and your eye. If a drop misses your eye, try again.
See Figure D.
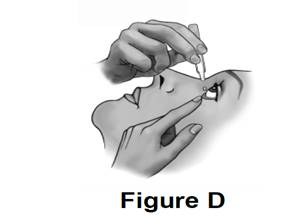
● If your doctor has told you to use tafluprost ophthalmic solution drops in both eyes, repeat Steps 7 to 10 for your other eye.
● There is enough tafluprost ophthalmic solution in one single-dose container for both of your eyes.
● Throw away the opened single-dose container with any remaining tafluprost ophthalmic solution right away.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811-7193
Made in France
Rx Only
Revised: 04/2024

- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TAFLUPROST
tafluprost solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50742-339 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAFLUPROST (UNII: 1O6WQ6T7G3) (TAFLUPROST - UNII:1O6WQ6T7G3) TAFLUPROST 0.015 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50742-339-30 6 in 1 CARTON 12/18/2024 1 NDC: 50742-339-05 5 in 1 POUCH 1 0.3 mL in 1 VIAL, SINGLE-DOSE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218002 12/18/2024 Labeler - Ingenus Pharmaceuticals, LLC (833250017) Registrant - Ingenus Pharmaceuticals, LLC (833250017) Establishment Name Address ID/FEI Business Operations Kaysersberg Pharmaceuticals 267486052 ANALYSIS(50742-339) , LABEL(50742-339) , MANUFACTURE(50742-339) , PACK(50742-339)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


