STRIVERDI RESPIMAT- olodaterol respimat inhalation spray spray, metered
Striverdi Respimat by
Drug Labeling and Warnings
Striverdi Respimat by is a Prescription medication manufactured, distributed, or labeled by Boehringer Ingelheim Pharmaceuticals, Inc., Boehringer Ingelheim Pharma GmbH and Co. KG. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use STRIVERDI RESPIMAT safely and effectively. See full prescribing information for STRIVERDI RESPIMAT.
STRIVERDI® RESPIMAT® (olodaterol) Inhalation Spray, for oral inhalation use
Initial U.S. Approval: 2014RECENT MAJOR CHANGES
INDICATIONS AND USAGE
STRIVERDI RESPIMAT Inhalation Spray is a long-acting beta2-adrenergic agonist (LABA) indicated for:
The long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema (1.1)
Important limitations:
DOSAGE AND ADMINISTRATION
- For oral inhalation only
- Two inhalations of STRIVERDI RESPIMAT once-daily at the same time of day (2)
DOSAGE FORMS AND STRENGTHS
Inhalation spray: Each actuation from the mouthpiece delivers 2.5 mcg olodaterol, equivalent to 2.7 mcg olodaterol hydrochloride. Two actuations equal one dose. (3)
CONTRAINDICATIONS
Use of a LABA, including STRIVERDI RESPIMAT, without an inhaled corticosteroid is contraindicated in patients with asthma. (4)
WARNINGS AND PRECAUTIONS
- LABA as monotherapy (without an inhaled corticosteroid) for asthma increases the risk of serious asthma-related events. (5.1)
- Do not initiate STRIVERDI RESPIMAT in acutely deteriorating COPD patients (5.2)
- Do not use for relief of acute symptoms. Concomitant short-acting beta2-agonists can be used as needed for acute relief. (5.2)
- Do not exceed the recommended dose. Excessive use of STRIVERDI RESPIMAT, or use in conjunction with other medications containing LABA can result in clinically significant cardiovascular effects and may be fatal. (5.3)
- Life-threatening paradoxical bronchospasm can occur. Discontinue STRIVERDI RESPIMAT immediately. (5.4)
- Use with caution in patients with cardiovascular or convulsive disorders, thyrotoxicosis or sensitivity to sympathomimetic drugs (5.5, 5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2% and more than placebo) are nasopharyngitis, upper respiratory tract infection, bronchitis, urinary tract infection, cough, dizziness, rash, diarrhea, back pain and arthralgia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or (800) 459-9906 TTY, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Other adrenergic drugs may potentiate effect. Use with caution. (5.3, 7.1)
- Xanthine derivatives, steroids, diuretics or non-potassium sparing diuretics may potentiate hypokalemia or ECG changes. Use with caution. (7.2, 7.3)
- MAO inhibitors, tricyclic antidepressants, and drugs that prolong QTc interval may potentiate effect on cardiovascular system. Use with extreme caution. (7.4)
- Beta-blockers may decrease effectiveness. Use with caution and only when medically necessary. (7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of COPD
1.2 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
5.2 Deterioration of Disease and Acute Episodes
5.3 Excessive Use of STRIVERDI RESPIMAT and Use with Long-Acting Beta2-Agonists
5.4 Paradoxical Bronchospasm
5.5 Cardiovascular Effects
5.6 Co-existing Conditions
5.7 Hypokalemia and Hyperglycemia
5.8 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
7 DRUG INTERACTIONS
7.1 Adrenergic Drugs
7.2 Xanthine Derivatives, Steroids, or Diuretics
7.3 Non-Potassium Sparing Diuretics
7.4 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
7.5 Beta-Blockers
7.6 Inhibitors of Cytochrome P450 and P-gp Efflux Transporter
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of COPD
STRIVERDI RESPIMAT is a long-acting beta2-agonist indicated for long-term, once-daily maintenance bronchodilator treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
1.2 Important Limitations of Use
STRIVERDI RESPIMAT is not indicated to treat acute deteriorations of COPD [see Warnings and Precautions (5.2)].
STRIVERDI RESPIMAT is not indicated to treat asthma. The safety and effectiveness of STRIVERDI RESPIMAT in asthma have not been established.
-
2 DOSAGE AND ADMINISTRATION
The recommended dose of STRIVERDI RESPIMAT is two inhalations once-daily at the same time of the day. Do not use STRIVERDI RESPIMAT more than two inhalations every 24 hours.
Prior to first use, the STRIVERDI RESPIMAT cartridge is inserted into the STRIVERDI RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
No dosage adjustment is required for geriatric patients, patients with mild and moderate hepatic impairment, or renally-impaired patients. There are no data available for use of STRIVERDI RESPIMAT in severe hepatically impaired patients [see Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
STRIVERDI RESPIMAT consists of a STRIVERDI RESPIMAT inhaler and an aluminum cylinder (STRIVERDI RESPIMAT cartridge) containing olodaterol (as the hydrochloride). The STRIVERDI RESPIMAT cartridge is intended for use with the STRIVERDI RESPIMAT inhaler only.
Each actuation from the STRIVERDI RESPIMAT inhaler delivers 2.5 mcg olodaterol, equivalent to 2.7 mcg olodaterol hydrochloride, from the mouthpiece. Two actuations equal one dose.
-
4 CONTRAINDICATIONS
Use of a LABA, including STRIVERDI RESPIMAT, without an inhaled corticosteroid is contraindicated in patients with asthma [see Warnings and Precautions (5.1)]. STRIVERDI RESPIMAT is not indicated for the treatment of asthma.
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events – Hospitalizations, Intubations, Death
- The safety and efficacy of STRIVERDI RESPIMAT in patients with asthma have not been established. STRIVERDI RESPIMAT is not indicated for the treatment of asthma [see Contraindications (4)].
- Use of long-acting beta2-adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed‑dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of LABA, including STRIVERDI RESPIMAT.
- No study adequate to determine whether the rate of asthma-related death is increased in patients treated with STRIVERDI RESPIMAT has been conducted.
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
5.2 Deterioration of Disease and Acute Episodes
STRIVERDI RESPIMAT should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. STRIVERDI RESPIMAT has not been studied in patients with acutely deteriorating COPD. The use of STRIVERDI RESPIMAT in this setting is inappropriate.
STRIVERDI RESPIMAT should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. STRIVERDI RESPIMAT has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
When beginning STRIVERDI RESPIMAT, patients who have been taking inhaled, short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing STRIVERDI RESPIMAT, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If STRIVERDI RESPIMAT no longer controls symptoms of bronchoconstriction, or the patient’s inhaled, short-acting beta2-agonist becomes less effective or the patient needs more inhalation of short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dosage of STRIVERDI RESPIMAT beyond the recommended dose is not appropriate in this situation.
5.3 Excessive Use of STRIVERDI RESPIMAT and Use with Long-Acting Beta2-Agonists
As with other inhaled drugs containing beta2-adrenergic agents, STRIVERDI RESPIMAT should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing long-acting beta2-agonists, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
5.4 Paradoxical Bronchospasm
As with other inhaled beta2-agonists, STRIVERDI RESPIMAT may produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs, STRIVERDI RESPIMAT should be discontinued immediately and alternative therapy instituted.
5.5 Cardiovascular Effects
STRIVERDI RESPIMAT, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and/or symptoms. If such effects occur, STRIVERDI RESPIMAT may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Long acting beta2-adrenergic agonists should be administered with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, hypertrophic obstructive cardiomyopathy, and hypertension.
5.6 Co-existing Conditions
STRIVERDI RESPIMAT, like other sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis, in patients with known or suspected prolongation of the QT interval, and in patients who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
5.7 Hypokalemia and Hyperglycemia
Beta-adrenergic agonists may produce significant hypokalemia in some patients, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Inhalation of high doses of beta2-adrenergic agonists may produce increases in plasma glucose.
In patients with severe COPD, hypokalemia may be potentiated by hypoxia and concomitant treatment [see Drug Interactions (7.2)], which may increase the susceptibility for cardiac arrhythmias.
Clinically notable decreases in serum potassium or changes in blood glucose were infrequent during clinical studies with long-term administration of STRIVERDI RESPIMAT with the rates similar to those for placebo controls. STRIVERDI RESPIMAT has not been investigated in patients whose diabetes mellitus is not well controlled.
-
6 ADVERSE REACTIONS
Long-acting beta2-adrenergic agonists, such as STRIVERDI RESPIMAT, as monotherapy (without an inhaled corticosteroid) for asthma, increase the risk of asthma-related events. STRIVERDI RESPIMAT is not indicated for the treatment of asthma [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience in Chronic Obstructive Pulmonary Disease
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The STRIVERDI RESPIMAT clinical development program included seven dose-ranging trials and eight confirmatory trials. Four of the confirmatory trials were 6-week cross-over trials and four were 48-week parallel group trials. Adverse reactions observed in the dose-ranging trials and four 6-week cross-over trials were consistent with those observed in the 48-week parallel group trials, which formed the primary safety database.
The primary safety database consisted of pooled data from the four 48-week double-blind, active and placebo-controlled, parallel group confirmatory clinical trials. These trials included 3104 adult COPD patients (77% males and 23% females) 40 years of age and older. Of these patients, 876 and 883 patients were treated with STRIVERDI RESPIMAT 5 mcg and 10 mcg once-daily, respectively. The STRIVERDI RESPIMAT groups were composed of mostly Caucasians (66%) with a mean age of 64 years and a mean percent predicted FEV1 at baseline of 44% for both the 5 mcg and 10 mcg treatment groups. Control arms for comparison included placebo in all four trials plus formoterol 12 mcg in two trials.
In these four clinical trials, seventy-two percent (72%) of patients exposed to any dose of STRIVERDI RESPIMAT reported an adverse reaction compared to 71% in the placebo group. The proportion of patients who discontinued due to an adverse reaction was 7.2% for STRIVERDI RESPIMAT treated patients compared to 8.8% for placebo treated patients. The adverse reaction most commonly leading to discontinuation was worsening COPD. The most common serious adverse reactions were COPD exacerbation, pneumonia, and atrial fibrillation.
Table 1 shows all adverse drug reactions reported by at least 2% of patients (and higher than placebo) who received STRIVERDI RESPIMAT 5 mcg during the 48-week trials.
Table 1 Number and frequency of adverse drug reactions greater than 2% (and higher than placebo) in COPD patients exposed to STRIVERDI RESPIMAT 5 mcg: Pooled data from the four 48-week, double-blind, active- and placebo-controlled clinical trials in COPD patients 40 years of age and older *Rash includes a grouping of similar terms Treatment STRIVERDI
5 mcg once-dailyPlacebo Body system (adverse drug reaction) n=876
n (%)n=885
n (%)Infections and infestations Nasopharyngitis 99 (11.3) 68 (7.7) Upper Respiratory Tract Infection 72 (8.2) 66 (7.5) Bronchitis 41 (4.7) 32 (3.6) Urinary Tract Infection 22 (2.5) 9 (1.0) Respiratory, thoracic, and mediastinal disorders Cough 37 (4.2) 35 (4.0) Nervous system disorders Dizziness 20 (2.3) 19 (2.1) Skin and subcutaneous tissue disorders Rash* 19 (2.2) 10 (1.1) Gastrointestinal disorders Diarrhea 25 (2.9) 22 (2.5) Musculoskeletal and connective tissue disorders Back Pain 31 (3.5) 24 (2.7) Arthralgia 18 (2.1) 7 (0.8) Additional adverse reactions that occurred in greater than 2% (and higher than placebo) of patients exposed to STRIVERDI RESPIMAT 10 mcg were pneumonia, constipation, and pyrexia.
Lung cancers were reported in 6 (0.7%), 3 (0.3%), and 2 (0.2%) patients who received STRIVERDI RESPIMAT 10 mcg, 5 mcg, and placebo, respectively.
-
7 DRUG
INTERACTIONS
7.1 Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of STRIVERDI RESPIMAT may be potentiated [see Warnings and Precautions (5.3, 5.5, 5.6, 5.7)].
7.2 Xanthine Derivatives, Steroids, or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of STRIVERDI RESPIMAT [see Warnings and Precautions (5.7)].
7.3 Non-Potassium Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of beta-agonists with non-potassium-sparing diuretics.
7.4 Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
STRIVERDI RESPIMAT, as with other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may be associated with an increased risk of ventricular arrhythmias.
7.5 Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and STRIVERDI RESPIMAT may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g. as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
7.6 Inhibitors of Cytochrome P450 and P-gp Efflux Transporter
In a drug interaction study using the strong dual CYP and P-gp inhibitor ketoconazole, a 1.7-fold increase of maximum plasma concentrations and AUC was observed [see Clinical Pharmacology (12.3)]. STRIVERDI RESPIMAT was evaluated in clinical trials for up to one year at doses up to twice the recommended therapeutic dose. No dose adjustment is necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled clinical studies with STRIVERDI RESPIMAT in pregnant women to inform of drug-associated risk of adverse pregnancy-related outcomes. There are clinical considerations with the use of STRIVERDI RESPIMAT in pregnant women [see Clinical Considerations]. Based on animal studies, olodaterol was not teratogenic when administered to pregnant rats or rabbits during organogenesis at inhalation doses of approximately 2731 or 1353 times the maximum recommended human daily inhalation dose (MRHDID) (on an AUC basis) in rats or rabbits, respectively [see Data].The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor and Delivery
There are no adequate and well-controlled human studies that have investigated the effects of STRIVERDI RESPIMAT during labor and delivery. Because of the potential for beta-agonist interference with uterine contractility, use of STRIVERDI RESPIMAT during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.Data
Animal Data
STRIVERDI RESPIMAT was not teratogenic in rats at inhalation doses approximately 2731 times the MRHDID (on an AUC basis at a maternal inhalation dose of 1054 mcg/kg/day). No significant effects occurred in rabbits at inhalation doses approximately 1353 times the MRHDID in adults (on an AUC basis at a maternal inhalation dose of 974 mcg/kg/day). Placental transfer of olodaterol was observed in pregnant rats.Olodaterol has been shown to be teratogenic in New Zealand rabbits at inhalation doses approximately 7130 times the MRHDID in adults (on an AUC basis at a maternal inhalation dose of 2489 mcg/kg/day). STRIVERDI RESPIMAT exhibited the following fetal toxicities: enlarged or small heart atria or ventricles, eye abnormalities, and split or distorted sternum.
8.2 Lactation
Risk Summary
There are no available data on the presence of olodaterol in human milk, the effects on the breastfed infant, or the effects on milk production. Olodaterol, the active component of STRIVERDI RESPIMAT, and/or its metabolites are present in the milk of lactating rats, however, due to species-specific differences in lactation physiology, the clinical relevance of these data are not clear [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for STRIVERDI RESPIMAT and any potential adverse effects on the breastfed child from STRIVERDI RESPIMAT or from the underlying maternal condition.Data
The distribution of olodaterol into milk was investigated after a single intravenous administration of 0.4 μmol/kg to lactating rats. Olodaterol and/or its metabolites are present in the milk of lactating rats at concentrations above those in plasma.8.4 Pediatric Use
STRIVERDI RESPIMAT is not indicated for use in children. The safety and effectiveness of STRIVERDI RESPIMAT in the pediatric population have not been established.
8.5 Geriatric Use
Based on available data, no adjustment of STRIVERDI RESPIMAT dosage in geriatric patients is necessary.
Of the 876 patients who received STRIVERDI RESPIMAT at the recommended dose of 5 mcg once-daily in the clinical studies from the pooled 1-year database, 485 were less than or equal to 65 years of age and 391 (44.6%) were greater than 65 years of age.
No overall differences in effectiveness were observed, and in the 1-year pooled data, the adverse drug reaction profiles were similar in the older population compared to the patient population overall.
-
10 OVERDOSAGE
The expected signs and symptoms with overdosage of STRIVERDI RESPIMAT are those of excessive beta-adrenergic stimulation and occurrence or exaggeration of any of the signs and symptoms, e.g., myocardial ischemia, angina pectoris, hypertension or hypotension, tachycardia, arrhythmias, palpitations, dizziness, nervousness, insomnia, anxiety, headache, tremor, dry mouth, muscle spasms, nausea, fatigue, malaise, hypokalemia, hyperglycemia, and metabolic acidosis. As with all inhaled sympathomimetic medications, cardiac arrest and even death may be associated with an overdose of STRIVERDI RESPIMAT.
Treatment of overdosage consists of discontinuation of STRIVERDI RESPIMAT together with institution of appropriate symptomatic and supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of STRIVERDI RESPIMAT. Cardiac monitoring is recommended in cases of overdosage.
-
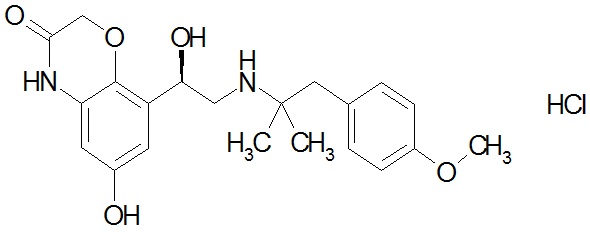
11 DESCRIPTION
The active moiety olodaterol is a selective beta2-adrenergic bronchodilator. The drug substance, olodaterol hydrochloride, is chemically described as 2H-1,4-Benzoxazin-3H(4H)-one, 6-hydroxy-8-[(1R)-1-hydroxy-2-[[2-(4-methoxyphenyl)-1,1-dimethylethyl]-amino]ethyl]-, monohydrochloride. Olodaterol hydrochloride is a white to off-white powder that is sparingly-slightly soluble in water and slightly soluble in ethanol. The molecular weight is 422.9 g/mole (salt): 386.5 g/mole (base), and the molecular formula is C21H26N2O5 x HCl as a hydrochloride. The conversion factor from salt to free base is 1.094.
The drug product, STRIVERDI RESPIMAT, is composed of a sterile, aqueous solution of olodaterol hydrochloride filled into a 4.5 mL plastic container crimped into an aluminum cylinder (STRIVERDI RESPIMAT cartridge) for use with the STRIVERDI RESPIMAT inhaler.
Excipients include water for injection, benzalkonium chloride, edetate disodium, and anhydrous citric acid. The STRIVERDI RESPIMAT cartridge is only intended for use with the STRIVERDI RESPIMAT inhaler. The STRIVERDI RESPIMAT inhaler is a hand held, pocket sized oral inhalation device that uses mechanical energy to generate a slow-moving aerosol cloud of medication from a metered volume of the drug solution. The STRIVERDI RESPIMAT inhaler has a yellow-colored cap.
When used with the STRIVERDI RESPIMAT inhaler, each cartridge containing a minimum of 4 grams of a sterile aqueous solution delivers the labeled number of metered actuations after preparation for use. Each dose (1 dose equals 2 actuations) from the STRIVERDI RESPIMAT inhaler delivers 5 mcg olodaterol in 22.1 mcL of solution from the mouthpiece. As with all inhaled drugs, the actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system. The duration of inspiration should be at least as long as the spray duration (1.5 seconds).
Prior to first use, the STRIVERDI RESPIMAT cartridge is inserted into the STRIVERDI RESPIMAT inhaler and the unit is primed. When using for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olodaterol is a long-acting beta2-adrenergic agonist (LABA). The compound exerts its pharmacological effects by binding and activation of beta2-adrenoceptors after topical administration by inhalation. Activation of these receptors in the airways results in a stimulation of intracellular adenyl cyclase, an enzyme that mediates the synthesis of cyclic-3’, 5’ adenosine monophosphate (cAMP). Elevated levels of cAMP induce bronchodilation by relaxation of airway smooth muscle cells. In vitro studies have shown that olodaterol has 241-fold greater agonist activity at beta2-adrenoceptors compared to beta1-adrenoceptors and 2,299-fold greater agonist activity compared to beta3-adrenoceptors. The clinical significance of these findings is unknown.
Beta-adrenoceptors are divided into three subtypes: beta1-adrenoceptors predominantly expressed on cardiac smooth muscle, beta2-adrenoceptors predominantly expressed on airway smooth muscle, and beta3-adrenoceptors predominantly expressed on adipose tissue. Beta2-agonists cause bronchodilation. Although the beta2-adrenoceptor is the predominant adrenergic receptor in the airway smooth muscle, it is also present on the surface of a variety of other cells, including lung epithelial and endothelial cells and in the heart. The precise function of beta2-receptors in the heart is not known, but their presence raises the possibility that even highly selective beta2-agonists may have cardiac effects.
12.2 Pharmacodynamics
Systemic Safety
The major adverse effects of inhaled beta2-adrenergic agonists occur as a result of excessive activation of systemic beta-adrenergic receptors. The most common adverse effects in adults include skeletal muscle tremor and cramps, insomnia, tachycardia, decreases in serum potassium, and increases in plasma glucose.Changes in serum potassium were evaluated in COPD patients in double-blind phase 3 studies. In pooled data, at the recommended 5 mcg dose there was no clinically relevant change compared to placebo in serum potassium.
Cardiac Electrophysiology
The effect of STRIVERDI RESPIMAT on the QT/QTc interval of the ECG was investigated in 24 healthy male and female volunteers in a double-blind, randomized, placebo- and active (moxifloxacin)- controlled study at single doses of 10, 20, 30, and 50 mcg. Dose-dependent QTcI (individual subject corrected QT interval) prolongation was observed. The maximum mean (one-sided 95% upper confidence bound) difference in QTcI from placebo after baseline correction was 2.5 (5.6) ms, 6.1 (9.2) ms, 7.5 (10.7) ms and 8.5 (11.6) ms following doses of 10, 20, 30 and 50 mcg, respectively.The effect of 5 mcg and 10 mcg STRIVERDI RESPIMAT on heart rate and rhythm was assessed using continuous 24-hour ECG recording (Holter monitoring) in a subset of 772 patients in the 48-week, placebo-controlled phase 3 trials. There were no dose- or time-related trends or patterns observed for the magnitudes of mean changes in heart rate or premature beats. Shifts from baseline to the end of treatment in premature beats did not indicate meaningful differences between STRIVERDI RESPIMAT 5 mcg, 10 mcg, and placebo.
12.3 Pharmacokinetics
Olodaterol showed linear pharmacokinetics. On repeated once-daily inhalation steady-state of olodaterol plasma concentrations was achieved after 8 days, and the extent of exposure was increased up to 1.8-fold as compared to a single dose.
Absorption
Olodaterol reaches maximum plasma concentrations generally within 10 to 20 minutes following drug inhalation. In healthy volunteers, the absolute bioavailability of olodaterol following inhalation was estimated to be approximately 30%, whereas the absolute bioavailability was below 1% when given as an oral solution. Thus, the systemic availability of olodaterol after inhalation is mainly determined by lung absorption, while any swallowed portion of the dose only negligibly contributes to systemic exposure.Distribution
Olodaterol exhibits multi-compartmental disposition kinetics after inhalation as well as after intravenous administration. The volume of distribution is high (1110 L), suggesting extensive distribution into tissue. In vitro binding of [14C] olodaterol to human plasma proteins is independent of concentration and is approximately 60%.Elimination
Total clearance of olodaterol in healthy volunteers is 872 mL/min, and renal clearance is 173 mL/min. The terminal half-life following intravenous administration is 22 hours. The terminal half-life following inhalation in contrast is about 45 hours, indicating that the latter is determined by absorption rather than by elimination processes. However, the effective half-life at daily dose of 5 μg calculated from Cmax from COPD patients is 7.5 hours.Metabolism
Olodaterol is substantially metabolized by direct glucuronidation and by O-demethylation at the methoxy moiety followed by conjugation. Of the six metabolites identified, only the unconjugated demethylation product binds to beta2-receptors. This metabolite, however, is not detectable in plasma after chronic inhalation of the recommended therapeutic dose.Cytochrome P450 isozymes CYP2C9 and CYP2C8, with negligible contribution of CYP3A4, are involved in the O-demethylation of olodaterol, while uridine diphosphate glycosyl transferase isoforms UGT2B7, UGT1A1, 1A7, and 1A9 were shown to be involved in the formation of olodaterol glucuronides.
Excretion
Following intravenous administration of [14C]-labeled olodaterol, 38% of the radioactive dose was recovered in the urine and 53% was recovered in feces. The amount of unchanged olodaterol recovered in the urine after intravenous administration was 19%. Following oral administration, only 9% of olodaterol and/or its metabolites was recovered in urine, while the major portion was recovered in feces (84%). More than 90% of the dose was excreted within 6 and 5 days following intravenous and oral administration, respectively. Following inhalation, excretion of unchanged olodaterol in urine within the dosing interval in healthy volunteers at steady state accounted for 5% to 7% of the dose.Specific Populations
A pharmacokinetic meta-analysis showed that no dose adjustment is necessary based on the effect of age, gender, and weight on systemic exposure in COPD patients after inhalation of STRIVERDI RESPIMAT.Patients with Renal Impairment
Olodaterol levels were increased by approximately 40% in subjects with severe renal impairment. A study in subjects with mild and moderate renal impairment was not performed.Patients with Hepatic Impairment
Subjects with mild and moderate hepatic impairment showed no changes in Cmax or AUC, nor did protein binding differ between mild and moderate hepatically impaired subjects and their healthy controls. A study in subjects with severe hepatic impairment was not performed.Drug Interaction Studies
Drug interaction studies were carried out using fluconazole as a model inhibitor of CYP 2C9 and ketoconazole as a potent P-gp (and CYP3A4, 2C8, 2C9) inhibitor.Fluconazole: Co-administration of 400 mg fluconazole once a day for 14 days had no relevant effect on systemic exposure to olodaterol.
Ketoconazole: Co-administration of 400 mg ketoconazole once a day for 14 days increased olodaterol Cmax by 66% and AUC0-1 by 68%.
Tiotropium: Co-administration of tiotropium bromide, delivered as fixed-dose combination with olodaterol, for 21 days had no relevant effect on systemic exposure to olodaterol, and vice versa.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year inhalation studies were conducted in rats and mice to assess the carcinogenic potential of olodaterol. Lifetime treatment of female rats induced leiomyomas of the mesovarium at doses of 25.8 and 270 mcg/kg/day (approximately 18- and 198-fold, respectively, the MRHDID on an AUC basis). No tumor findings were observed in male rats at doses up to 270 mcg/kg/day (approximately 230-fold the MRHDID on an AUC basis). Lifetime treatment of female mice induced leiomyomas and leiomyosarcomas of the uterus at doses ≥76.9 mcg/kg/day (approximately 106-fold the MRHDID on an AUC basis). No tumor findings were observed in male mice at doses up to 255 mcg/kg/day (approximately 455-fold the MRHDID on an AUC basis). Increases in leiomyomas and leiomyosarcomas of the female rodent reproductive tract have been similarly demonstrated with other β2-adrenergic agonist drugs. The relevance of these findings to human use is unknown.
Olodaterol was not mutagenic in the in vitro Ames test or in the in vitro mouse lymphoma assay. Olodaterol produced increased frequency of micronuclei in rats after intravenous doses. The increased frequency of micronuclei was likely related to drug enhanced (compensatory) erythropoiesis. The mechanism for induction of micronuclei formation is likely not relevant at clinical exposures.
Olodaterol did not impair male or female fertility in rats at inhalation doses up to 3,068 mcg/kg/day (approximately 2,322 times the MRHDID on an AUC basis).
-
14 CLINICAL STUDIES
The STRIVERDI RESPIMAT clinical development program included three dose-ranging trials in COPD patients, four dose-ranging trials in asthma patients, and eight confirmatory trials in patients with COPD.
Dose-ranging trials
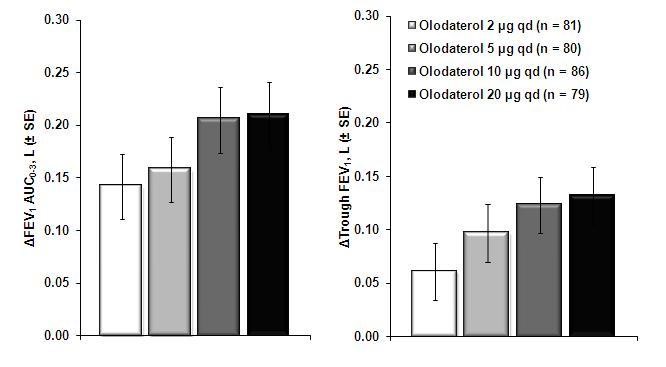
The first COPD dose-ranging trial was a randomized, double-blind, placebo-controlled, single-dose, 5-way cross-over trial in 36 patients. Results demonstrated dose-related improvements in forced expiratory volume in one second (FEV1) compared to placebo. The difference in trough FEV1 from placebo for the 2, 5, 10, and 20 mcg doses were 0.07L (95% CI 0.03, 0.11), 0.10L (0.06, 0.14), 0.11L (0.07, 0.15), and 0.12L (0.08, 0.16), respectively. The second COPD dose-ranging trial was a 4-week, randomized, double-blind, placebo-controlled, parallel group trial in 405 patients. Dose-related improvements in lung function were also seen, with no added benefit of the 20 mcg dose over the 10 mcg dose (Figure 1). The third COPD dose-ranging trial was a randomized, double-blind, 4-way cross-over, dose-regimen trial in 47 patients. Treatment arms included 2 mcg twice-daily, 5 mcg once-daily, 5 mcg twice-daily, and 10 mcg once-daily. There was no clear difference in treatment effect when comparing twice-daily dosing to once-daily dosing.Figure 1 Difference from placebo for STRIVERDI RESPIMAT for FEV1 AUC0-3hr and trough FEV1 after 4 weeks
Four randomized, double-blind, placebo-controlled dose-ranging trials were performed in patients with asthma, evaluating doses from 2 to 20 mcg. Results from patients with asthma were consistent with results from dose-ranging trials in patients with COPD. STRIVERDI RESPIMAT is not indicated for asthma.
Based upon the results of the dose-ranging trials, 5 and 10 mcg doses were further evaluated in the confirmatory COPD trials.
Confirmatory Trials
The eight confirmatory trials in the STRIVERDI RESPIMAT clinical development program were four pairs of replicate, randomized, double-blind, placebo-controlled trials in 3533 COPD patients (1281 received the 5 mcg dose, 1284 received the 10 mcg dose):
(i) two replicate, placebo-controlled, parallel group, 48 week trials (Trials 1 and 2)
(ii) two replicate, placebo- and active- [formoterol 12 mcg twice-daily] controlled, parallel group, 48-week trials (Trials 3 and 4)
(iii) two replicate, placebo- and active- [formoterol 12 mcg twice-daily] controlled, 6-week cross-over trials (Trials 5 and 6)
(iv) two replicate, placebo- and active- [tiotropium bromide 18 mcg once-daily] controlled, 6-week cross-over trials (Trials 7 and 8).These eight trials enrolled patients who were 40 years of age or older with a clinical diagnosis of COPD, a smoking history of at least 10 pack-years, and moderate to very severe pulmonary impairment (post-bronchodilator FEV1 less than 80% predicted normal [GOLD II – IV] and a post-bronchodilator FEV1 to FVC ratio of less than 70%).
The majority of the 3104 patients in the 48-week trials (Trials 1 and 2, Trials 3 and 4) were male (77%), white (66%) or Asian (32%), with a mean age of 64 years. Mean post-bronchodilator FEV1 was 1.38 L (GOLD II [50%], GOLD III [40%], GOLD IV [10%]). Mean beta2-agonist responsiveness was 15% of baseline (0.16 L). With the exception of other LABAs, all pulmonary medications were allowed as concomitant therapy (e.g., tiotropium [24%], ipratropium [25%], inhaled corticosteroids [45%], xanthines [16%]); patient enrollment was stratified by tiotropium use. In all four trials, the primary efficacy endpoints were change from pre-treatment baseline in FEV1 AUC0-3 and trough (pre-dose) FEV1 (after 12 weeks in Trials 1 and 2; after 24 weeks in Trials 3 and 4).
In all four 48-week trials, STRIVERDI RESPIMAT 5 mcg demonstrated significant improvements in FEV1 AUC0-3hr compared to placebo at week 12 (Table 2) and at week 24. In the four 48-week trials, STRIVERDI RESPIMAT 5 mcg demonstrated significant improvements in trough FEV1 compared to placebo at week 12 (Table 2; 3 of 4 trials) and at week 24 (4 trials). STRIVERDI RESPIMAT 5 mcg demonstrated a bronchodilatory treatment effect at 5 minutes after the first dose with a mean increase in FEV1 compared to placebo of 0.11L (range: 0.10L to 0.12L). The 10 mcg dose demonstrated no additional benefit over the 5 mcg dose (data not shown). Patients treated with STRIVERDI RESPIMAT 5 mcg used less rescue albuterol compared to patients treated with placebo.
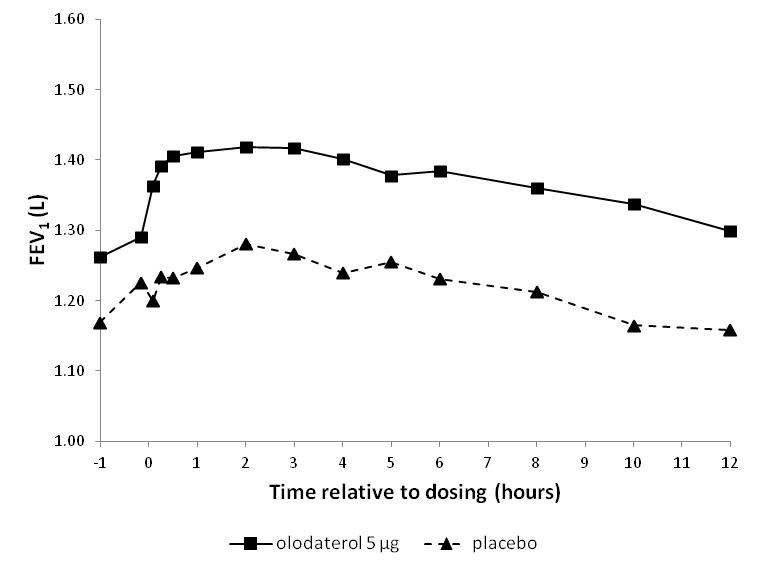
Table 2 Differences from placebo for STRIVERDI RESPIMAT 5 mcg for FEV1 AUC0-3hr and trough FEV1 at week 12 Difference from placebo [L] (95% CI) FEV1 AUC0-3hr Trough FEV1 Trial 1 0.16 (0.12, 0.21) 0.08 (0.04, 0.13) Trial 2 0.13 (0.09, 0.18) 0.03 (-0.01, 0.08) Trial 3 0.18 (0.14, 0.22) 0.08 (0.04, 0.12) Trial 4 0.15 (0.11, 0.18) 0.06 (0.02, 0.10) In Trials 1 and 2, serial spirometric evaluations were performed pre-dose and up to 12 hours after dosing in a sub-group of 562 patients (201 patients receiving STRIVERDI RESPIMAT 5 mcg, 192 patients receiving 10 mcg, and 169 patients receiving placebo) after 12 weeks of treatment. Dosing occurred at approximately the same time of the day in the morning. The spirometric curves from Trial 1 are displayed in Figure 2.
Figure 2 FEV1 profile for STRIVERDI RESPIMAT 5 mcg and placebo at week 12 (pre-dose and up to 12 hrs post-dose) (Trial 1)
The bronchodilatory profile of STRIVERDI RESPIMAT 5 mcg over the 24 hour dosing interval was evaluated in two pairs of replicate, placebo- and active-controlled, 6 week cross-over trials in 199 patients (Trials 5 and 6) and 230 patients (Trials 7 and 8) with moderate to very severe COPD. Mean beta2-agonist responsiveness ranged from 14% -21% of baseline (0.18 to 0.22 L). All pulmonary medications were allowed as concomitant therapy with the exception of other LABAs (all trials) and anti-cholinergics (Trials 7 and 8). In all four trials, the primary endpoints were change from pre-treatment baseline in FEV1 AUC0-12hr and FEV1 AUC12-24hr after 6 weeks; although not a primary endpoint, trough FEV1 was also measured after 6 weeks. Results are shown in Table 3.
Table 3 Differences from placebo for STRIVERDI RESPIMAT 5 mcg after 6 weeks in cross-over spirometry trials Difference from placebo [L] (95% CI) FEV1 AUC0-12hr FEV1 AUC12-24 hr Trough FEV1 Trial 5 0.15 (0.11, 0.18) 0.11 (0.07, 0.15) 0.11 (0.06, 0.15) Trial 6 0.17 (0.14, 0.21) 0.12 (0.08, 0.15) 0.10 (0.05, 0.15) Trial 7 0.19 (0.15, 0.23) 0.13 (0.09, 0.17) 0.13 (0.10, 0.17) Trial 8 0.20 (0.16, 0.23) 0.15 (0.12, 0.19) 0.13 (0.10, 0.17) -
16 HOW
SUPPLIED/STORAGE AND HANDLING
STRIVERDI RESPIMAT Inhalation Spray is supplied in a labeled carton containing one STRIVERDI RESPIMAT cartridge and one STRIVERDI RESPIMAT inhaler.
The STRIVERDI RESPIMAT cartridge is an aluminum cylinder with a tamper protection seal on the cap. The STRIVERDI RESPIMAT cartridge is only intended for use with the STRIVERDI RESPIMAT inhaler.
The STRIVERDI RESPIMAT inhaler is a cylindrical-shaped plastic inhalation device with a gray-colored body and a clear base. The clear base is removed to insert the cartridge. The inhaler contains a dose indicator. The yellow colored cap and the written information on the label of the gray inhaler body indicates that it is labeled for use with the STRIVERDI RESPIMAT cartridge.
STRIVERDI RESPIMAT Inhalation Spray is available as:
STRIVERDI RESPIMAT Inhalation Spray: 60 metered actuations (NDC: 0597-0192-61)The STRIVERDI RESPIMAT cartridge has a net fill weight of at least 4 grams and when used with the STRIVERDI RESPIMAT inhaler, is designed to deliver the labeled number of metered actuations after preparation for use.
When the labeled number of metered actuations has been dispensed from the inhaler, the STRIVERDI RESPIMAT locking mechanism will be engaged and no more actuations can be dispensed.
After assembly, the STRIVERDI RESPIMAT inhaler should be discarded at the latest 3 months after first use or when the locking mechanism is engaged, whichever comes first.
Keep out of reach of children. Do not spray into eyes.
Storage
Store at 25°C (77°F); excursions permitted to 15°C–30°C (59°F–86°F) [see USP Controlled Room Temperature]. Avoid freezing. -
17 PATIENT COUNSELING
INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Asthma-Related Events
Inform patients that LABA, such as STRIVERDI RESPIMAT, when used as monotherapy [without an inhaled corticosteroid], increase the risk of serious asthma-related events, including asthma-related death. STRIVERDI RESPIMAT is not indicated for the treatment of asthma.Not for Acute Symptoms
STRIVERDI RESPIMAT is not meant to relieve acute asthma symptoms or exacerbations of COPD and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist such as albuterol. (The healthcare provider should provide the patient with such medication and instruct the patient in how it should be used.)Instruct patients to notify their physician immediately if they experience any of the following:
- Worsening of symptoms
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- Significant decrease in lung function as outlined by the physician
Instruct patients not to stop therapy with STRIVERDI RESPIMAT without physician/provider guidance since symptoms may recur after discontinuation.
Do Not Use Additional Long-Acting Beta2-Agonists
Patients who have been taking inhaled, short-acting beta2-agonists on a regular basis should be instructed to discontinue the regular use of these products and use them only for the symptomatic relief of acute symptoms.When patients are prescribed STRIVERDI RESPIMAT, other inhaled medications containing long-acting beta2-agonists should not be used. Patients should not use more than the recommended once-daily dose of STRIVERDI RESPIMAT. Excessive use of sympathomimetics may cause significant cardiovascular effects, and may be fatal.
Risks Associated with Beta2-Agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness.Paradoxical Bronchospasm
Inform patients that STRIVERDI RESPIMAT can produce paradoxical bronchospasm. Advise patients that if paradoxical bronchospasm occurs, patients should discontinue STRIVERDI RESPIMAT.Hypersensitivity Reactions
Inform patients that immediate hypersensitivity reactions, including angioedema, may occur after administration of STRIVERDI RESPIMAT. Advise patient to immediately discontinue STRIVERDI RESPIMAT and consult a physician.Instructions for Administering STRIVERDI RESPIMAT
It is important for patients to understand how to correctly administer STRIVERDI RESPIMAT inhalation spray using the STRIVERDI RESPIMAT inhaler. Instruct patients that STRIVERDI RESPIMAT inhalation spray should only be administered via the STRIVERDI RESPIMAT inhaler and the STRIVERDI RESPIMAT inhaler should not be used for administering other medications.Instruct patients that priming STRIVERDI RESPIMAT is essential to ensure appropriate content of the medication in each actuation.
When using the unit for the first time, the STRIVERDI RESPIMAT cartridge is inserted into the STRIVERDI RESPIMAT inhaler and the unit is primed. STRIVERDI RESPIMAT patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 05/2019 PATIENT INFORMATION
STRIVERDI® RESPIMAT® (STRIH ver dee– RES peh mat)
(olodaterol)
inhalation spray, for oral inhalation useWhat is STRIVERDI RESPIMAT? - STRIVERDI RESPIMAT is a long-acting beta2-adrenergic agonist (LABA) medicine, olodaterol.
- LABA medicines such as STRIVERDI RESPIMAT help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- STRIVERDI RESPIMAT is for long-term use and should be taken as 2 puffs 1 time each day to improve the symptoms of COPD for better breathing.
- STRIVERDI RESPIMAT is a prescription medicine used to control the symptoms of COPD in adults with COPD. COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- STRIVERDI RESPIMAT is not used to treat sudden symptoms of COPD. Always have a beta2-agonist inhaler medicine (rescue inhaler) with you to treat sudden symptoms of COPD. If you do not have a rescue inhaler medicine, contact your healthcare provider to have one prescribed for you.
- STRIVERDI RESPIMAT is not for the treatment of asthma. It is not known if STRIVERDI RESPIMAT is safe and effective in people with asthma.
- STRIVERDI RESPIMAT should not be used in children. It is not known if STRIVERDI RESPIMAT is safe and effective in children.
Do not use STRIVERDI RESPIMAT if you have asthma. Before you use STRIVERDI RESPIMAT, tell your healthcare provider about all of your medical conditions, including if you: - have heart problems.
- have high blood pressure.
- have seizures.
- have thyroid problems.
- have diabetes.
- are pregnant or plan to become pregnant. It is not known if the medicine olodaterol in STRIVERDI RESPIMAT can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if the medicine olodaterol in STRIVERDI RESPIMAT passes into your breast milk and if it can harm your baby. You and your healthcare provider should decide if you will take STRIVERDI RESPIMAT while breastfeeding.
- are allergic to any of the ingredients in STRIVERDI RESPIMAT, any other medicines, or food products.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine.How should I use STRIVERDI RESPIMAT?
Read the step-by-step instructions for using STRIVERDI RESPIMAT at the end of this Patient Information leaflet.- Do not use STRIVERDI RESPIMAT unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly. Ask your healthcare provider or pharmacist if you have any questions.
- The STRIVERDI RESPIMAT inhaler has a slow moving mist that helps you inhale the medicine.
- Use STRIVERDI RESPIMAT exactly as your healthcare provider tells you to use it. Do not use STRIVERDI RESPIMAT more often than prescribed.
- Use 1 dose (2 puffs) of STRIVERDI RESPIMAT, 1 time each day, at the same time of the day.
- If you miss a dose of STRIVERDI RESPIMAT, take it as soon as you remember. Do not take more than 1 dose (2 puffs) in 24 hours.
- If you take too much STRIVERDI RESPIMAT, call your healthcare provider or go to the nearest hospital emergency room right away.
- Do not spray STRIVERDI RESPIMAT in your eyes.
- STRIVERDI RESPIMAT Inhalation Spray should only be given using the STRIVERDI RESPIMAT inhaler. The STRIVERDI RESPIMAT inhaler should not be used to give other medicines.
- Always use the new STRIVERDI RESPIMAT inhaler that is provided with each new prescription.
- STRIVERDI RESPIMAT does not relieve sudden symptoms of COPD. You should not take extra doses of STRIVERDI RESPIMAT to relieve sudden symptoms of COPD. Always have a rescue inhaler medicine with you to treat sudden symptoms. If you do not have a rescue inhaler medicine, call your healthcare provider to have one prescribed for you.
- Do not stop using STRIVERDI RESPIMAT or other medicines to control or treat your COPD unless told to do so by your healthcare provider because your symptoms might get worse. Your healthcare provider will change your medicines as needed.
- If your COPD symptoms worsen over time do not increase your dose of STRIVERDI RESPIMAT, instead call your healthcare provider.
-
Do not use STRIVERDI RESPIMAT:
- more often than prescribed for you,
- at a higher dose than prescribed for you, or
- with other LABA medicines. Ask your healthcare provider or pharmacist if any of your other medicines are LABA medicines.
- Call your healthcare provider or get emergency medical care right away if your breathing problems worsen with STRIVERDI RESPIMAT, you need to use your rescue inhaler medicine more often than usual, or your rescue inhaler medicine does not work as well for you at relieving your symptoms.
What are the possible side effects of STRIVERDI RESPIMAT?
STRIVERDI RESPIMAT can cause serious side effects, including:- serious problems from asthma. People with asthma who take long-acting beta2-adrenergic agonist (LABA) medicines, such as STRIVERDI RESPIMAT, without also using a medicine called an inhaled corticosteroid, have an increased risk of serious problems from asthma, including being hospitalized, needing a tube placed in their airway to help them breathe, or death.
-
call your healthcare provider if breathing problems
worsen over time while using STRIVERDI RESPIMAT. You may need
a different treatment.
Get emergency medical care if:- your breathing problems worsen quickly
- you use your rescue inhaler medicine, but it does not relieve your breathing problems.
- using too much of a LABA medicine may cause:
- chest pain
- fast and irregular heartbeat
- tremor
- increased blood pressure
- headache
- nervousness
- COPD symptoms can get worse over time. If your COPD symptoms worsen over time, do not increase your dose of STRIVERDI RESPIMAT, instead call your healthcare provider.
- sudden shortness of breath can happen immediately after using STRIVERDI RESPIMAT. Sudden shortness of breath may be life-threatening. Stop taking STRIVERDI RESPIMAT and call your healthcare provider or get emergency medical help right away if you get sudden shortness of breath after using STRIVERDI RESPIMAT.
- effects on your heart, including fast or irregular heartbeat, palpitations, chest pain or increased blood pressure.
- changes in laboratory blood levels including high blood sugar (hyperglycemia) and low levels of potassium (hypokalemia), which may cause symptoms of muscle weakness or abnormal heart rhythm.
- serious allergic reactions including rash, hives, itching, swelling of the face, lips, tongue, throat, difficulties in breathing or swallowing. Stop taking STRIVERDI RESPIMAT and get emergency medical help right away if you get any symptoms of a serious allergic reaction after using STRIVERDI RESPIMAT.
These are not all the side effects of STRIVERDI RESPIMAT. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store STRIVERDI RESPIMAT? - Store STRIVERDI RESPIMAT at room temperature, between 68°F to 77°F (20°C to 25°C).
- Do not freeze your STRIVERDI cartridge or RESPIMAT inhaler.
- Keep STRIVERDI RESPIMAT inhaler, cartridge, and all medicines out of the reach of children.
General information about safe and effective use of STRIVERDI RESPIMAT.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use STRIVERDI RESPIMAT for a condition for which it was not prescribed. Do not give STRIVERDI RESPIMAT to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about STRIVERDI RESPIMAT that is written for health professionals.Active ingredient: olodaterol
Inactive ingredients: water for injection, benzalkonium chloride, edetate disodium, and anhydrous citric acid
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
STRIVERDI® and RESPIMAT® are registered trademarks and are used under license from Boehringer Ingelheim International GmbH
Copyright © 2019 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
IT5644EF042019
This product’s label may have been updated. For current full prescribing information for STRIVERDI RESPIMAT, go to www.STRIVERDI.com or to view a video demonstration on how to use your STRIVERDI RESPIMAT inhaler, scan the code below or visit www.themist.com. You may also call 1-800-542-6257 or (TTY) 1-800-459-9906 for further information about STRIVERDI RESPIMAT.

-
INSTRUCTIONS FOR USE
Instructions for Use
STRIVERDI® RESPIMAT® (STRIH ver dee- RES peh mat)
(olodaterol)
inhalation spray, for oral inhalation useFor Oral Inhalation Only
Do not spray STRIVERDI RESPIMAT into your eyes.
Read these Instructions for Use before you start using STRIVERDI RESPIMAT and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
You will need to use this inhaler 1 time each day, at the same time each day. Each time you use it take 2 puffs.
Do not turn the clear base before inserting the cartridge.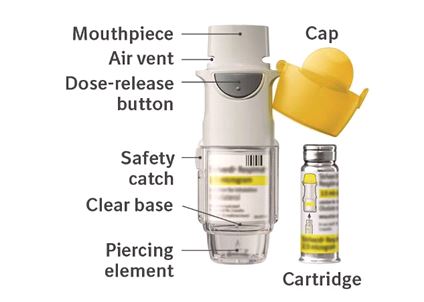 How to store your STRIVERDI
RESPIMAT inhaler
How to store your STRIVERDI
RESPIMAT inhaler
- Store STRIVERDI RESPIMAT at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze your STRIVERDI RESPIMAT cartridge and inhaler.
- If STRIVERDI RESPIMAT has not been used for more than 3 days, release 1 puff towards the ground.
- If STRIVERDI RESPIMAT has not been used for more than 21 days, repeat steps 4 to 6 under the “Prepare for first use” until a mist is visible. Then repeat steps 4 to 6 three more times.
- Keep your STRIVERDI RESPIMAT cartridge and inhaler out of the reach of children.
Clean the mouthpiece, including the metal part inside the mouthpiece, with a damp cloth or tissue only, at least once a week. Any minor discoloration in the mouthpiece does not affect your STRIVERDI RESPIMAT inhaler.
When to get a new STRIVERDI RESPIMAT inhaler- Your inhaler contains 60 puffs (30 doses) if used as indicated (2 puffs once daily). If you have a sample, your inhaler contains 28 puffs (14 doses) if used as indicated (2 puffs once daily).

- The dose indicator shows approximately how much medicine is left.
- When the dose indicator enters the red area of the scale you need to get a refill; there is approximately medicine for 7 days left (if you have a sample, there is approximately medicine for 3 days left).
- When the dose indicator reaches the end of the red scale, your STRIVERDI RESPIMAT is empty and automatically locks. At this point, the clear base cannot be turned any further.
- Three months after insertion of cartridge, throw away the STRIVERDI RESPIMAT even if it has not been used, or when the inhaler is locked, or when it expires, whichever comes first.
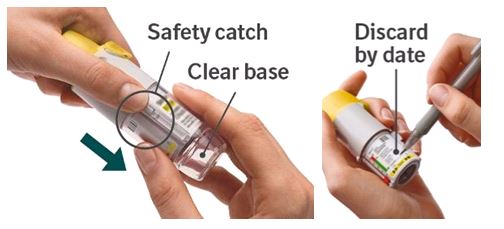
1. Remove clear base - Keep the cap closed.
- Press the safety catch while firmly pulling off the clear base with your other hand. Be careful not to touch the piercing element.
- Write the discard by date on the label (3 months from the date the cartridge is inserted).
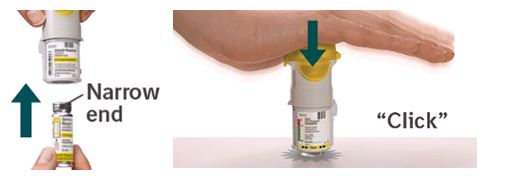
2. Insert cartridge - Insert the narrow end of the cartridge into the inhaler.
- Place the inhaler on a firm surface and push down firmly until it clicks into place.
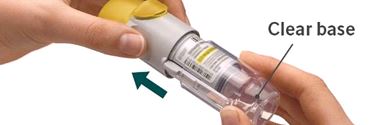
3. Replace clear base - Put the clear base back into place until it clicks.
- Do not remove the clear base or the cartridge after it has been put together.
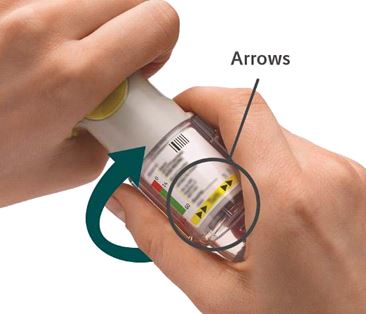
4. Turn - Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).

5. Open - Open the cap until it snaps fully open.

6. Press - Point the inhaler toward the ground.
- Press the dose-release button.
- Close the cap.
- If you do not see a mist, repeat steps 4 to 6 until a mist is seen.
- After a mist is seen, repeat steps 4 to 6 three more times.
- After complete preparation of your inhaler, it will be ready to deliver the number of puffs on the label.

Turn - Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).
Open - Open the cap until it snaps fully open.

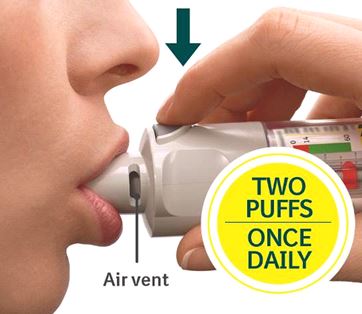
Press - Breathe out slowly and fully.
- Close your lips around the mouthpiece without covering the air vents.
- Point the inhaler to the back of your throat.
- While taking a slow, deep breath through your mouth, Press the dose-release button and continue to breathe in.
- Hold your breath for 10 seconds or for as long as comfortable.
- Repeat Turn, Open, Press (TOP) for a total of 2 puffs.
- Close the cap until you use your inhaler again.
It is difficult to insert the cartridge deep enough:
Did you accidentally turn the clear base before inserting the cartridge? Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first? Insert the cartridge with the narrow end first.
I cannot press the dose-release button:
Did you turn the clear base? If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the STRIVERDI RESPIMAT pointing to zero? The STRIVERDI RESPIMAT inhaler is locked after 60 puffs (30 doses). If you have a sample, the STRIVERDI RESPIMAT inhaler is locked after 28 puffs (14 doses). Prepare and use your new STRIVERDI RESPIMAT inhaler.
Did you turn the clear base already? If the clear base has already been turned, follow steps “Open” and “Press” under “Daily use” to get your medicine.
Is the dose indicator on the STRIVERDI RESPIMAT pointing to zero? The STRIVERDI RESPIMAT inhaler is locked after 60 puffs (30 doses). If you have a sample, the STRIVERDI RESPIMAT inhaler is locked after 28 puffs (14 doses). Prepare and use your new STRIVERDI RESPIMAT inhaler.
The dose indicator on the STRIVERDI RESPIMAT reaches zero too soon:
Did you use STRIVERDI RESPIMAT as indicated (2 puffs once daily)? STRIVERDI RESPIMAT will deliver 60 puffs and last 30 days if used at 2 puffs once daily. If you have a sample, STRIVERDI RESPIMAT will deliver 28 puffs and last 14 days if used at 2 puffs once daily.
Did you turn the clear base before you inserted the cartridge? The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the STRIVERDI RESPIMAT is working? Once you have prepared STRIVERDI RESPIMAT, no test-spraying is required if used daily.
Did you insert the cartridge into a used STRIVERDI RESPIMAT? Always insert a new cartridge into a NEW STRIVERDI RESPIMAT.
My STRIVERDI RESPIMAT sprays automatically:
Was the cap open when you turned the clear base? Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base? Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked? Turn the clear base in a continuous movement until it clicks (half a turn).
My STRIVERDI RESPIMAT doesn’t spray:
Did you insert a cartridge? If not, insert a cartridge.
Did you repeat Turn, Open, Press (TOP) less than three times after inserting the cartridge? Repeat Turn, Open, Press (TOP) three times after inserting the cartridge as shown in steps 4 to 6 under “Prepare for first use”.
Is the dose indicator on the STRIVERDI RESPIMAT pointing to 0? You have used up all your medicine and the inhaler is locked.
This product’s label may have been updated. For current full prescribing information for STRIVERDI RESPIMAT, go to www.STRIVERDI.com or to view a video demonstration on how to use your STRIVERDI RESPIMAT inhaler, scan the code below or visit www.themist.com. You may also call 1-800-542-6257 or (TTY) 1-800-459-9906 for further information about STRIVERDI RESPIMAT.

This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
STRIVERDI® and RESPIMAT® are registered trademarks and are used under license from Boehringer Ingelheim International GmbH
Copyright © 2019 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STRIVERDI RESPIMAT
olodaterol respimat inhalation spray spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0597-0192 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Olodaterol hydrochloride (UNII: 65R445W3V9) (Olodaterol - UNII:VD2YSN1AFD) Olodaterol 2.5 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0597-0192-61 1 in 1 CARTON 08/01/2014 1 60 in 1 CARTRIDGE; Type 0: Not a Combination Product 2 NDC: 0597-0192-28 1 in 1 CARTON 08/01/2014 03/31/2017 2 28 in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203108 08/01/2014 Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH and Co. KG 551147440 MANUFACTURE(0597-0192) , PACK(0597-0192) , ANALYSIS(0597-0192) , API MANUFACTURE(0597-0192)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.