OMEGAVEN- fish oil injection, emulsion
Omegaven by
Drug Labeling and Warnings
Omegaven by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Fresenius Kabi Austria. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMEGAVEN safely and effectively. See full prescribing information for OMEGAVEN.
OMEGAVEN (fish oil triglycerides) injectable emulsion, for intravenous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC). (1)
Limitations of Use:
- Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in parenteral nutrition (PN)-dependent patients. (1)
- It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6:omega-3 fatty acid ratio of the product. (1)
DOSAGE AND ADMINISTRATION
- For infusion into a central or peripheral vein. (2.1)
- May be infused directly from the bottle or admixed in a parenteral nutrition (PN) container. (2.1, 2.2)
- Prior to administration, correct severe fluid and electrolyte disorders and measure serum triglycerides to establish a baseline level. (2.3)
- Initiate dosing in PN-dependent pediatric patients as soon as direct or conjugated bilirubin levels are 2 mg/dL or greater. (2.3)
- Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize, and consideration of additional energy sources given to the patient. (2.3)
- The recommended daily dose (and the maximum dose) in pediatric patients is 1 g/kg/day. (2.3)
- For information on infusion rate when initiating dosing and in patients with elevated triglyceride levels, see the full prescribing information. (2.3)
- The recommended duration for infusion is between 8 and 24 hours, depending on the clinical situation. (2.3)
- Administer Omegaven until direct or conjugated bilirubin levels are less than 2 mg/dL or until the patient no longer requires PN. (2.3)
DOSAGE FORMS AND STRENGTHS
Injectable Emulsion: 5 g/50 mL and 10 g/100 mL (0.1 g/mL) in a single-dose bottle. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of Death in Preterm Infants due to Pulmonary Lipid Accumulation: Deaths in preterm infants after infusion of intravenous soybean oil-based lipid emulsions have been reported in literature, and autopsy findings included intravascular lipid accumulation in the lungs. Risk with Omegaven is unknown. Monitor for signs and symptoms of pleural or pericardial effusion. (5.1)
- Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reaction occurs. (5.2)
- Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters. (5.3, 5.4, 5.5, 5.6)
- Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm infants. (5.7)
- Monitoring and Laboratory Tests: Routine laboratory monitoring is recommended, including monitoring for essential fatty acid deficiency. (5.8)
ADVERSE REACTIONS
Most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Antiplatelet Agents and Anticoagulants: Prolonged bleeding time has been reported in patients taking antiplatelet agents or anticoagulants and oral omega-3 fatty acids. Periodically monitor bleeding time in patients receiving Omegaven and concomitant antiplatelet agents or anticoagulants. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
2.2 Admixing Instructions
2.3 Dosing Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Death in Preterm Infants due to Pulmonary Lipid Accumulation
5.2 Hypersensitivity Reactions
5.3 Risk of Infections
5.4 Fat Overload Syndrome
5.5 Refeeding Syndrome
5.6 Hypertriglyceridemia
5.7 Aluminum Toxicity
5.8 Monitoring and Laboratory Tests
5.9 Interference with Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antiplatelet Agents and Anticoagulants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Limitations of Use:
- Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in parenteral nutrition (PN)-dependent patients [see Clinical Studies (14)].
- It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6:omega-3 fatty acid ratio of the product [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
- Omegaven can be administered alone or as part of a PN admixture.
- Omegaven is for central or peripheral intravenous infusion. When administered with dextrose and amino acids, the choice of a central or peripheral venous route should depend on the osmolarity of the final infusate. Solutions with osmolarity of 900 mOsm/L or greater must be infused through a central vein.
- Use a 1.2 micron in-line filter during administration.
- Use a dedicated line for PN. Omegaven should be infused concurrently into the same vein as dextrose-amino acid solutions (as part of PN) by a Y-connector located closest to the infusion site; flow rates of each solution should be controlled separately by infusion pumps. Avoid multiple connections; do not connect multiple medications in series. Turn off the pump before the bottle runs dry.
- Use a vented infusion set when Omegaven is infused from the bottle.
- Do not use infusion sets and lines that contain di-2-ethylhexyl phthalate (DEHP). Infusion sets that contain polyvinyl chloride (PVC) components have DEHP as a plasticizer.
- Prior to infusion, visually inspect Omegaven for particulate matter and discoloration. Discard the bottle if any particulates or discoloration are observed.
- Gently invert the bottle before use. Use Omegaven only if the emulsion is homogeneous and the container is undamaged.
- Strict aseptic techniques must be followed.
- Hang the bottle using the attached hanger and start infusion.
- After connecting the infusion set, start infusion of Omegaven immediately. Complete the infusion within 12 hours when using a Y-connector and within 24 hours when used as part of an admixture.
- For single use only. Discard unused portion.
2.2 Admixing Instructions
If Omegaven is administered as part of a PN admixture, follow the instructions below.
- Prepare the admixture in PN containers using strict aseptic techniques to avoid microbial contamination.
- Do not add Omegaven directly to the empty PN container; destabilization of the lipid emulsion may occur.
- When Omegaven is administered with other infusion solutions (e.g., amino acids, dextrose), the compatibility of the solutions used must be ensured. Questions related to compatibility may be directed to Fresenius Kabi USA, LLC, at 1-800-551-7176.
- The following proper mixing sequence must be followed to minimize pH-related problems by ensuring that typically acidic dextrose solutions are not mixed with lipid emulsions alone:
- Transfer dextrose solution to the PN container.
- Transfer amino acid solution to the PN container.
- Transfer Omegaven to the PN container.
Simultaneous transfer of amino acid solution, dextrose solution, and Omegaven using an automated compounding device is also permitted; follow automated compounding device instructions as indicated.
Use gentle agitation during admixing to minimize localized concentration effects; shake container gently after each addition.
- The prime destabilizers of emulsions are excessive acidity (such as a pH less than 5) and inappropriate electrolyte content. Care should be taken if adding divalent cations (e.g., Ca++ and Mg++), which have been shown to cause emulsion instability. Amino acid solutions exert buffering effects that can protect the emulsion from destabilization.
- Inspect the admixture to ensure that precipitates have not formed during preparation of the admixture and the emulsion has not separated. Separation of the emulsion can be visibly identified by a yellowish streaking or the accumulation of yellowish droplets in the admixture. Discard the admixture if any of these are observed.
- The remaining contents of a partly used PN container must be discarded.
- Start infusion of admixtures containing Omegaven immediately. If not used immediately, admixtures may be stored for up to 6 hours at room temperature or up to 24 hours under refrigeration. Complete the infusion within 24 hours after removal from storage.
- Follow the instructions of each product included in the admixture.
2.3 Dosing Information
Dosing Considerations
- Prior to administration of Omegaven, correct severe fluid and electrolyte disorders and measure serum triglycerides to establish a baseline level.
- Initiate Omegaven dosing as soon as direct or conjugated bilirubin (DBil) levels are 2 mg/dL or greater in pediatric patients who are expected to be PN-dependent for at least 2 weeks.
- The dosing of Omegaven depends on each patient's energy requirements, which may be influenced by age, body weight, tolerance, clinical status, and ability to metabolize and eliminate lipids.
- When determining dose, take into account the energy supplied by dextrose and amino acids from PN, as well as energy from oral or enteral nutrition. Energy provided from lipid-based medications must also be taken into account (e.g., propofol).
- Omegaven contains 0.15 to 0.30 mg/mL of dl-alpha-tocopherol. Take into account the amount of alpha-tocopherol in Omegaven when determining the need for additional supplementation of vitamin E.
Recommended Pediatric Dosing
- The recommended Omegaven dosage for pediatric patients is 1 g/kg/day; this is also the maximum daily dose.
- The initial rate of infusion should not exceed 0.05 mL/minute for the first 15 to 30 minutes of infusion. If tolerated, gradually increase until reaching the required rate after 30 minutes. The maximum infusion rate should not exceed 1.5 mL/kg/hour, corresponding to 0.15 g/kg/hour.
- If hypertriglyceridemia (triglycerides greater than 250 mg/dL in neonates and infants or greater than 400 mg/dL in older children) develops once Omegaven has been initiated at the recommended dosage, consider stopping the administration of Omegaven for 4 hours and obtain a repeat serum triglyceride level. Resume Omegaven based on new result as indicated.
- In patients with elevated triglyceride levels, consider other reasons for hypertriglyceridemia (e.g., renal disease, other drugs). If triglycerides remain at elevated levels, consider a reduced dose of 0.5 g to 0.75 g/kg/day with an incremental increase to 1 g/kg/day.
- Monitor triglyceride levels during treatment [see Warnings and Precautions (5.6, 5.8)].
- The recommended duration for infusion of Omegaven is between 8 and 24 hours, depending on the clinical situation.
- Administer Omegaven until DBil levels are less than 2 mg/dL or until the patient no longer requires PN.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Use of Omegaven is contraindicated in patients with:
- Known hypersensitivity to fish or egg protein or to any of the active ingredients or excipients [see Warnings and Precautions (5.2)].
- Severe hemorrhagic disorders due to a potential effect on platelet aggregation.
- Severe hyperlipidemia or severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride concentrations greater than 1,000 mg/dL) [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Death in Preterm Infants due to Pulmonary Lipid Accumulation
Deaths in preterm infants after infusion of soybean oil-based intravenous lipid emulsions have been reported in medical literature. Autopsy findings in these preterm infants included intravascular lipid accumulation in the lungs. The risk of pulmonary lipid accumulation with Omegaven is unknown.
Preterm and small-for-gestational-age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion. This risk due to poor lipid clearance should be considered when administering intravenous lipid emulsions.
Monitor patients receiving Omegaven for signs and symptoms of pleural or pericardial effusion.
5.2 Hypersensitivity Reactions
Omegaven contains fish oil and egg phospholipids, which may cause hypersensitivity reactions. Signs or symptoms of a hypersensitivity reaction may include: tachypnea, dyspnea, hypoxia, bronchospasm, tachycardia, hypotension, cyanosis, vomiting, nausea, headache, sweating, dizziness, altered mentation, flushing, rash, urticaria, erythema, fever, or chills. If a hypersensitivity reaction occurs, stop infusion of Omegaven immediately and initiate appropriate treatment and supportive measures [see Contraindications (4)].
5.3 Risk of Infections
Lipid emulsions, such as Omegaven, can support microbial growth and are an independent risk factor for the development of bloodstream infections. The risk of infection is increased in patients with malnutrition-associated immunosuppression, long-term use and poor maintenance of intravenous catheters, or immunosuppressive effects of other conditions or concomitant drugs.
To decrease the risk of infectious complications, ensure aseptic technique in catheter placement and maintenance, as well as in the preparation and administration of Omegaven.
Monitor for signs and symptoms of early infections including fever and chills, laboratory test results that might indicate infection (including leukocytosis and hyperglycemia), and frequently inspect the intravenous catheter insertion site for edema, redness, and discharge.
5.4 Fat Overload Syndrome
Fat overload syndrome is a rare condition that has been reported with intravenous lipid emulsions. A reduced or limited ability to metabolize lipids accompanied by prolonged plasma clearance may result in this syndrome, which is characterized by a sudden deterioration in the patient's condition including fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, hepatomegaly, deteriorating liver function, and central nervous system manifestations (e.g., coma). The cause of fat overload syndrome is unclear. Although it has been most frequently observed when the recommended lipid dose was exceeded, cases have also been described where the lipid formulation was administered according to instructions. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
5.5 Refeeding Syndrome
Administering PN to severely malnourished patients may result in refeeding syndrome, which is characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, closely monitor severely malnourished patients and slowly increase their nutrient intake.
5.6 Hypertriglyceridemia
Impaired lipid metabolism with hypertriglyceridemia may occur in conditions such as inherited lipid disorders, obesity, diabetes mellitus, and metabolic syndrome. Serum triglyceride levels greater than 1,000 mg/dL have been associated with an increased risk of pancreatitis [see Contraindications (4)].
To evaluate the patient's capacity to metabolize and eliminate the infused lipid emulsion, measure serum triglycerides before the start of infusion (baseline value), and regularly throughout treatment.
If hypertriglyceridemia (triglycerides greater than 250 mg/dL in neonates and infants or greater than 400 mg/dL in older children) develops, consider stopping the administration of Omegaven for 4 hours and obtain a repeat serum triglyceride level. Resume Omegaven based on new result as indicated [see Dosage and Administration (2.3)].
5.7 Aluminum Toxicity
Omegaven contains no more than 25 mcg/L of aluminum. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Preterm infants are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Patients with impaired kidney function, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
5.8 Monitoring and Laboratory Tests
Routine Monitoring
Monitor serum triglycerides [see Warnings and Precautions (5.6)], fluid and electrolyte status, blood glucose, liver and kidney function, coagulation parameters, and complete blood count including platelets throughout treatment.
Essential Fatty Acids
Monitoring patients for laboratory evidence of essential fatty acid deficiency (EFAD) is recommended. Laboratory tests are available to determine serum fatty acids levels. Reference values should be consulted to help determine adequacy of essential fatty acid status. Increasing essential fatty acid intake (enterally or parenterally) is effective in treating and preventing EFAD.
5.9 Interference with Laboratory Tests
The lipids contained in Omegaven may interfere with some laboratory blood tests (e.g., hemoglobin, lactate dehydrogenase, bilirubin, and oxygen saturation) if blood is sampled before lipids have cleared from the bloodstream. Lipids are normally cleared after a period of 5 to 6 hours once the lipid infusion is stopped.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of death in preterm infants due to pulmonary lipid accumulation [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Risk of infections [see Warnings and Precautions (5.3)]
- Fat overload syndrome [see Warnings and Precautions (5.4)]
- Refeeding syndrome [see Warnings and Precautions (5.5)]
- Hypertriglyceridemia [see Warnings and Precautions (5.6)]
- Aluminum toxicity [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety database for Omegaven reflects exposure in 189 pediatric patients (19 days to 15 years of age) treated for a median of 14 weeks (3 days to 8 years) in two clinical trials. Omegaven was administered at a maximum dose of 1 g/kg/day as the lipid component of a PN regimen which also included dextrose, amino acids, vitamins, and trace elements; 158 (84%) of these patients received concurrent lipids from enteral nutrition [see Clinical Studies (14)].
Adverse reactions that occurred in more than 5% of patients who received Omegaven and with a higher incidence than the comparator group are shown in Table 1. Patients had a complicated medical and surgical history prior to receiving Omegaven treatment and the mortality was 13%. Underlying clinical conditions prior to the initiation of Omegaven therapy included prematurity, low birth weight, necrotizing enterocolitis, short bowel syndrome, ventilator dependence, coagulopathy, intraventricular hemorrhage, and sepsis.
Table 1 Adverse Reactions in Greater Than 5% of Omegaven-Treated Pediatric Patients with PNAC Adverse Reaction Omegaven
(N=189)
n (%)Vomiting 87 (46) Agitation 67 (35) Bradycardia 66 (35) Apnea 38 (20) Viral Infection 30 (16) Erythema 23 (12) Rash 15 (8) Abscess 14 (7) Neutropenia 13 (7) Hypertonia 11 (6) Incision site erythema 11 (6) Twelve (6%) Omegaven-treated patients were listed for liver transplantation (1 patient was listed 18 days before treatment, and 11 patients after a median of 42 days [range: 2 days to 8 months] of treatment); 9 (5%) received a transplant after a median of 121 days (range: 25 days to 6 months) of treatment, and 3 (2%) were taken off the waiting list because cholestasis resolved.
One hundred thirteen (60%) Omegaven-treated patients reached DBil levels less than 2 mg/dL and AST or ALT levels less than 3 times the upper limit of normal, with median AST and ALT levels for Omegaven-treated patients at 89 and 65 U/L, respectively, by the end of the study.
Median hemoglobin levels and platelet counts for Omegaven-treated patients at baseline were 10.2 g/dL and 173 × 109/L, and by the end of the study these levels were 10.5 g/dL and 217 × 109/L, respectively. Adverse reactions associated with bleeding were experienced by 74 (39%) of Omegaven-treated patients.
Median glucose levels at baseline and the end of the study were 86 and 87 mg/dL for Omegaven-treated patients, respectively. Hyperglycemia was experienced by 13 (7%) Omegaven-treated patients.
Median triglyceride levels at baseline and the end of the study were 121 mg/dL and 72 mg/dL for Omegaven-treated patients respectively. Hypertriglyceridemia was experienced by 5 (3%) Omegaven-treated patients.
The triene:tetraene (Mead acid:arachidonic acid) ratio was used to monitor essential fatty acid status in Omegaven-treated patients only in Study 1 (n = 123) [see Warnings and Precautions (5.8)]. The median triene:tetraene ratio was 0.02 (interquartile range: 0.01 to 0.03) at both baseline and the end of the study. Blood samples for analysis may have been drawn while the lipid emulsion was being infused and patients received enteral or oral nutrition.
6.2 Postmarketing Experience
The following adverse reaction has been identified with use of Omegaven in another country. Because this reaction was reported voluntarily from a population of uncertain size, it is not possible to reliably estimate its frequency or establish a causal relationship to drug exposure.
Life-threatening hemorrhage following a central venous catheter change was reported in a 9-month-old infant with intestinal failure who received PN with Omegaven as the sole lipid source; he had no prior history of bleeding, coagulopathy, or portal hypertension.
-
7 DRUG INTERACTIONS
7.1 Antiplatelet Agents and Anticoagulants
Some published studies have demonstrated prolongation of bleeding time in patients taking antiplatelet agents or anticoagulants and oral omega-3 fatty acids. The prolongation of bleeding times reported in those studies did not exceed normal limits and there were no clinically significant bleeding episodes. Nonetheless, it is recommended to periodically monitor bleeding time in patients receiving Omegaven and concomitant antiplatelet agents or anticoagulants.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Omegaven use in pregnant women to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with fish oil triglycerides.
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
No data are available regarding the presence of fish oil triglycerides from Omegaven in human milk, the effects on the breastfed infant, or the effects on milk production. Lactating women receiving oral omega-3 fatty acids have been shown to have higher levels of omega-3 fatty acids in their milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Omegaven, and any potential adverse effects of Omegaven on the breastfed infant.
8.4 Pediatric Use
The effectiveness of Omegaven was established in two open-label clinical trials of 82 pediatric patients, 3 to 42 weeks of age, including preterm neonates with estimated gestational age of greater than 24 weeks at birth. Patients administered Omegaven attained and maintained growth through at least 108 weeks of treatment [see Clinical Studies (14)].
The safety of Omegaven was established in 189 pediatric patients (19 days to 15 years of age). The most common adverse reactions in Omegaven-treated patients were vomiting, agitation, and bradycardia [(see Adverse Reactions (6.1)].
Deaths in preterm infants after infusion of intravenous soybean oil-based lipid emulsion have been reported in literature [see Warnings and Precautions (5.1)].
Preterm neonates and infants who receive treatment with Omegaven may be at risk of aluminum toxicity and other metabolic abnormalities [see Warnings and Precautions (5.7, 5.8)].
-
10 OVERDOSAGE
In the event of an overdose, fat overload syndrome may occur [see Warnings and Precautions (5.4)]. Stop the infusion of Omegaven until triglyceride levels have normalized and any symptoms have abated. The effects are usually reversible by stopping the lipid infusion. If medically appropriate, further intervention may be indicated. Lipids are not dialyzable from serum.
-
11 DESCRIPTION
Omegaven (fish oil triglycerides) is a sterile, nonpyrogenic, white, homogenous emulsion for intravenous infusion as a supply of calories in patients with PNAC. Each mL of Omegaven contains 0.1 g of fish oil, 0.012 g egg phospholipids, 0.025 g glycerin, 0.15 to 0.3 mg dl-alpha-tocopherol, 0.3 mg sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9). The phosphate content is 0.015 mmol/mL.
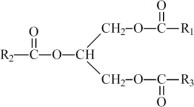
The fish oil included in Omegaven is a triglyceride mixture consisting of esters of long-chain saturated fatty acids and unsaturated fatty acids with the following structure:
where
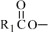 ,
, 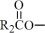 , and
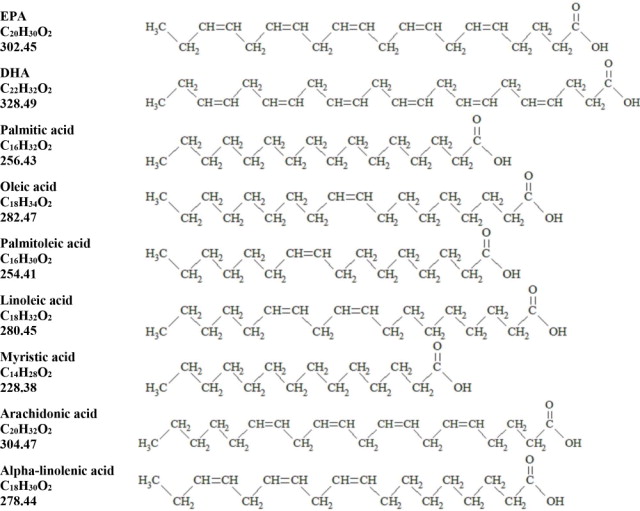
, and 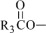 are long chain acyl groups. Because triglycerides often contain different long chain fatty acids at each position, possible structures can have molecular weights ranging from 700 to 1000 g/mol. The main fatty acid components of the fish oil in Omegaven are EPA (13% to 26%) and DHA (14% to 27%). The fish oil also contains palmitic acid (4% to 12%), oleic acid (4% to 11%), palmitoleic acid (4% to 10%), myristic acid (2% to 7%), and arachidonic acid (0.2% to 2.0%). Additionally, the mean contents of linoleic acid and alpha linolenic acid are 1.5% and 1.1%, respectively. The fish oil component has a total omega-3 fatty acid content of 40% to 54%. The empirical formula, molecular weight, and chemical structure of the main fatty acid components are:
are long chain acyl groups. Because triglycerides often contain different long chain fatty acids at each position, possible structures can have molecular weights ranging from 700 to 1000 g/mol. The main fatty acid components of the fish oil in Omegaven are EPA (13% to 26%) and DHA (14% to 27%). The fish oil also contains palmitic acid (4% to 12%), oleic acid (4% to 11%), palmitoleic acid (4% to 10%), myristic acid (2% to 7%), and arachidonic acid (0.2% to 2.0%). Additionally, the mean contents of linoleic acid and alpha linolenic acid are 1.5% and 1.1%, respectively. The fish oil component has a total omega-3 fatty acid content of 40% to 54%. The empirical formula, molecular weight, and chemical structure of the main fatty acid components are:
Omegaven 5 g/50 mL contains 5 grams of fish oil and 0.6 g egg phospholipids, 1.25 g glycerin, 7.5 to 15 mg dl-alpha-tocopherol, 0.015 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9) packaged in a single-dose 50-mL glass bottle enclosed with a rubber stopper. The phosphate content of the drug product is 0.75 mmol.
The mean content of the two major fatty acid components in 50 mL are 1.0 g EPA (range: 0.6 to 1.5 g) and 0.96 g DHA (range: 0.7 to 1.7 g). Additionally, the mean content of linoleic acid, alpha-linolenic acid, and arachidonic acid per 50 mL are 0.16 g, 0.07 g, and 0.13 g, respectively.
Omegaven 10 g/100 mL contains 10 grams of fish oil and 1.2 g egg phospholipids, 2.5 g glycerin, 15 to 30 mg dl-alpha-tocopherol, 0.03 g sodium oleate, water for injection, and sodium hydroxide for pH adjustment (pH 6 to 9) packaged in a single-dose 100-mL glass bottle enclosed with rubber stopper. The phosphate content of the drug product is 1.5 mmol. The mean content of the two major fatty acid components in 100 mL are 2.0 g EPA (range: 1.2 to 3.0 g) and 1.9 g DHA (range: 1.3 to 3.3 g). Additionally, the mean content of linoleic acid, alpha-linolenic acid, and arachidonic acid per 100 mL are 0.31g, 0.13 g, and 0.25 g; respectively.
The total energy content of Omegaven is 112 kcal/100 mL (1.12 kcal/mL), including lipids, phospholipids, and glycerol.
Omegaven has an osmolality of approximately 342 mOsm/kg water (which represents an osmolarity of 273 mOsm/L).
Omegaven contains no more than 25 mcg/L of aluminum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Omegaven provides a biologically utilizable source of calories and essential fatty acids.
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are also important for membrane structure and function, as precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
12.3 Pharmacokinetics
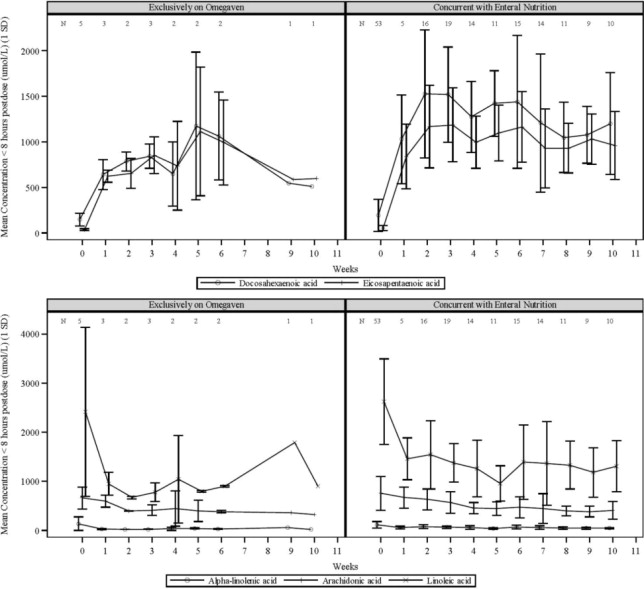
The plasma concentrations of EPA and DHA, the major fatty acids in Omegaven, as well as linoleic acid and alpha-linolenic acid (essential fatty acids) were measured along with the markers of essential fatty acid status in 58 pediatric patients with PNAC after an intravenous infusion of 1 mg/kg/day of Omegaven over 10 weeks. Five patients received Omegaven as the exclusive lipid source, and all others received concurrent enteral or oral nutrition.
Figure 1 Mean Plasma Concentrations of Fatty Acids Over 10 Weeks of Omegaven Infusion in Pediatric Patients with PNAC
Error bars represent ± 1 standard deviation (SD).
Numbers at the top of plots represent the number of patients at each time point
If more than one value was available for a patient at any given time point, the average was used.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed with fish oil triglycerides to evaluate the carcinogenic potential or its effect on fertility.
Fish oil triglycerides was negative in the bacterial mutagenicity test with Salmonella typhimurium and the hypoxanthine phosphoribosyl transferase (HPRT) gene mutation assay in Chinese hamster V79 cells. Fish oil triglycerides was not clastogenic in cultured human peripheral lymphocytes or in a rat bone marrow cytogenetic study.
-
14 CLINICAL STUDIES
The efficacy of Omegaven was evaluated in two open-label single-center clinical trials (Study 1, NCT00910104, and Study 2, NCT00738101) in pediatric patients with PNAC (defined as direct or conjugated bilirubin [DBil] equal to or greater than 2 mg/dL) who required PN for at least 14 days. Although Study 1 and Study 2 were not adequately designed to demonstrate noninferiority or superiority of Omegaven to the soybean oil-based lipid emulsion comparator, the data from these studies support Omegaven as a source of calories in pediatric patients with PNAC. Nutritional efficacy was assessed by biomarkers of lipid metabolism, growth indices (body weight, length/height and head circumference), and/or mean changes in fatty acid parameters.
Both trials prospectively enrolled Omegaven-treated patients (maximum dose of 1 g/kg/day) and used historical control patients who received a soybean oil-based lipid emulsion (maximum dose of 3 g/kg/day) as a comparator. Patients were expected to require PN, which also included dextrose, amino acids, vitamins and trace elements, for at least 30 days (Study 1) or 14 days (Study 2), had PNAC, and had received standard therapies to prevent progression of liver disease. Study 1 enrolled patients less than 2 years of age and Study 2 enrolled patients less than 5 years of age. Patients with another cause of chronic liver disease (in the absence of intestinal failure) were excluded. Patients with an international normalized ratio (INR) greater than 2 and patients with portal vein thrombosis or reversal of portal flow by abdominal ultrasound were also excluded.
For the efficacy analyses of Studies 1 and 2, Omegaven-treated patients were pair-matched in a 2:1 manner to historical control patients primarily based on DBil levels and postmenstrual age at baseline. There were 123 patients (82 Omegaven; 41 historical control) in this population, 78 (52; 26) were from Study 1, and 45 (30; 15) were from Study 2. A summary of concurrent enteral/oral nutrition intake for each study is provided in Table 2.
Table 2 Summary of Median Enteral or Oral Intakes in Pediatric Patients with PNAC in Study 1 and Study 2 a. Two Omegaven-treated patients in Study 1 did not have data regarding enteral or oral intakes.
Parameter Study 1 Study 2 Omegaven
(n=50)aHistorical Control
(n=26)Omegaven
(n=30)Historical Control
(n=15)Number of patients who received concurrent enteral or oral nutrition 44 (88%) 26 (100%) 24 (80%) 14 (93%) Percentage of total calories provided enterally or orally, median (Min - Max) 24%
(1% – 53%)25%
(0.4% – 68%)21%
(1% – 75%)12%
(3% – 40%)In the combined efficacy analysis population from Study 1 and Study 2, median chronological age was 9 weeks (range: 3 to 42 weeks) in the Omegaven group and 7 weeks (range: 0 to 41 weeks) in the historical control group. The majority of these patients were preterm infants at birth (90% Omegaven; 83% historical control), with gestational age categories as follows: extremely preterm (31%; 20%); very preterm (20%; 24%); moderate or late preterm (40%; 39%). A majority of patients were also considered to have low, very-low, or extremely-low birth weights (76%; 82%), with birth weight categories as follows: extremely-low birth weight (34%; 24%); very-low birth weight (17%; 21%); low birth weight (25%; 37%).
The efficacy analysis population had more males (51%; 59%) than females, and the majority of patients were White (60%; 66%).
At baseline, the median age-adjusted body weight (Z-score) was -1.3 for the Omegaven group and -1.1 for the historical control group; 27% and 28% were low-for-age in body weight, 43% and 40% were low-for-age in body height/length, and 25% and 15% were low-for-age in head circumference for the Omegaven and historical control groups, respectively (low-for-age corresponded to Z-scores less than or equal to -1.9 for each growth parameter). In the efficacy analysis population, baseline median DBil, AST, and ALT levels were 3.8 mg/dL, 101 U/L, and 67 U/L, respectively, for the Omegaven group; and 3.8 mg/dL, 115 U/L, and 52 U/L, respectively, for the historical control group.
The median (range) of the duration of treatment was 2.7 months (5 days to 8 years) for the Omegaven group and 3.6 months (16 days to 2 years) for the historical control group.
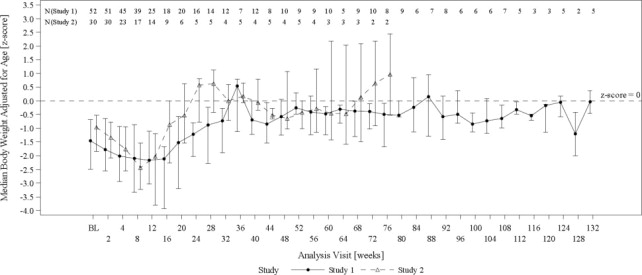
The changes in median age-adjusted body weight (Z-scores) over time for Omegaven-treated patients (Figure 2) appeared similar to those for historical control patients. In both the Omegaven and historical control groups, there was an initial decline in all growth parameters (weight, height/length, head circumference) over the initial weeks of treatment, followed by catch-up growth and more age-appropriate values through the remainder of the study. By comparing the Omegaven study data to age-standardized Fenton and World Health Organization (WHO) growth charts to assess age appropriate growth in patients with PNAC, patients treated with Omegaven as their exclusive lipid source also achieved age-appropriate growth.
Figure 2 Median Age-Adjusted Body Weight (Z-scores) Over Time in Omegaven-Treated Pediatric Patients with PNAC in Study 1 and Study 2*
BL = baseline
Error bars represent interquartile ranges.
*Data from pair-matched Omegaven patients were truncated at week 132. Median values are only shown for visits with data from at least 2 patients at a particular visit.
In the combined analysis from Study 1 and Study 2, the number of Omegaven and historical control patients who achieved full enteral feeding by the end of the study was 52 (63%) patients and 24 (59%) patients, respectively. The median time to full enteral feeding was approximately 15 weeks for both groups.
At the end of the studies, the median DBil level for Omegaven-treated patients was 0.60 mg/dL (interquartile range: 0.1 to 2.8 mg/dL). The Kaplan-Meier estimate of the median time for DBil values to return to less than 2.0 mg/dL was approximately 5.7 weeks [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Omegaven (fish oil triglycerides) injectable emulsion, 5 g/50 mL and 10 g/100 mL (0.1 g/mL) is a white, homogenous, sterile emulsion supplied as follows:
50 mL single-dose glass bottle NDC: 63323-205-21 Carton of 10 x 50 mL NDC: 63323-205-50 100 mL single-dose glass bottle NDC: 63323-205-31 Carton of 10 x 100 mL NDC: 63323-205-00 The stopper used as the bottle closure is not made with natural rubber latex, PVC, or DEHP.
Storage and Handling
Store below 25°C (77°F). Avoid excessive heat. Do not freeze. If accidentally frozen, discard product.
Once the bottle is connected to the infusion set, use Omegaven immediately. Complete infusion within 12 hours when using a Y-connector [see Dosage and Administration (2.1)].
Infuse admixtures containing Omegaven immediately. If not used immediately, admixtures can be stored for up to 6 hours at room temperature or up to 24 hours under refrigeration. Complete the infusion within 24 hours after removal from storage [see Dosage and Administration (2.2)].
-
17 PATIENT COUNSELING INFORMATION
Inform patients, their families, or caregivers of the following risks of Omegaven:
- Risk of death in preterm infants due to pulmonary lipid accumulation [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
- Risk of infections [see Warnings and Precautions (5.3)]
- Fat overload syndrome [see Warnings and Precautions (5.4)]
- Refeeding syndrome [see Warnings and Precautions (5.5)]
- Hypertriglyceridemia [see Warnings and Precautions (5.6)]
- Aluminum toxicity [see Warnings and Precautions (5.7)]
Fresenius Kabi and Omegaven are registered trademarks of Fresenius Kabi.
Manufactured by:
Graz, Austria
www.fresenius-kabi.com/us
451555 -
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 50 mL Vial Label
NDC: 63323-205-21 255050
Omegaven®
(fish oil triglycerides) Injectable emulsion
5 grams per 50 mL
(0.1 grams per mL)
Energy: 56 kcal per 50 mL
For intravenous use only. Rx only
50 mL Single-Dose bottle - Discard Unused Portion
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 50 mL Vial Carton Label
NDC: 63323-205-50 255050
Omegaven®
(fish oil triglycerides) Injectable emulsion
5 grams per 50 mL
(0.1 grams per mL)
For intravenous use only. Rx only
Sterile.
Store below 25°C. (77°F). Do not freeze.
Use only if the emulsion is homogeneous.
See prescribing information.
Not made with natural rubber latex
10 x 50 mL
Single-Dose bottle-
Discard Unused Portion
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 100 mL Vial Label
NDC: 63323-205-31 255100
Omegaven®
(fish oil triglycerides) Injectable emulsion
10 grams per 100 mL
(0.1 grams per mL)
Energy: 112 kcal per 100 mL
For intravenous use only. Rx only
100 mL Single-Dose bottle - Discard Unused Portion
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL- PRINCIPAL DISPLAY – Omegaven 100 mL Vial Carton Label
NDC: 63323-205-00 255100
Omegaven®
(fish oil triglycerides) Injectable emulsion
10 grams per 100 mL
(0.1 grams per mL)
For intravenous use only. Rx only
Sterile.
Store below 25°C. (77°F). Do not freeze.
Use only if the emulsion is homogeneous.
See prescribing information.
Not made with natural rubber latex
10 x 100 mL Single-Dose bottle-
Discard Unused Portion
-
INGREDIENTS AND APPEARANCE
OMEGAVEN
fish oil injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-205 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fish oil (UNII: XGF7L72M0F) (Fish oil - UNII:XGF7L72M0F) Fish oil 0.1 g in 1 mL Inactive Ingredients Ingredient Name Strength .alpha.-tocopherol, dl- (UNII: 7QWA1RIO01) egg phospholipids (UNII: 1Z74184RGV) sodium oleate (UNII: 399SL044HN) glycerin (UNII: PDC6A3C0OX) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-205-50 10 in 1 CARTON 07/27/2018 1 NDC: 63323-205-21 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC: 63323-205-00 10 in 1 CARTON 07/27/2018 2 NDC: 63323-205-31 100 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210589 07/27/2018 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Austria 300206604 MANUFACTURE(63323-205)
Trademark Results [Omegaven]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OMEGAVEN 78089074 2645876 Live/Registered |
FRESENIUS SE & CO. KGAA 2001-10-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.