BLISTEX COLD AND ALLERGY LIP SOOTHER- dimethicone and pramoxine hydrochloride stick
Blistex by
Drug Labeling and Warnings
Blistex by is a Otc medication manufactured, distributed, or labeled by Blistex Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
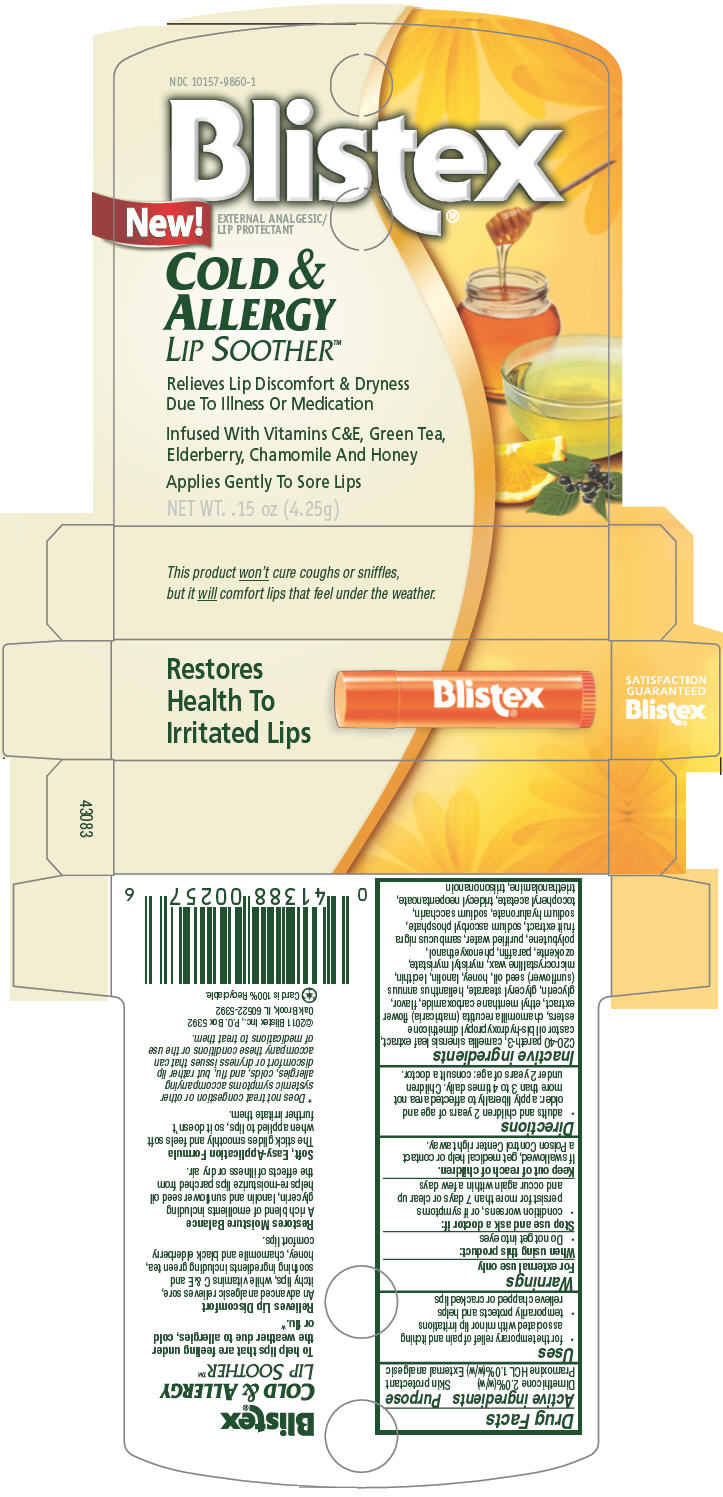
- Uses
- Warnings
- Directions
-
Inactive ingredients
C20-40 pareth-3, camellia sinensis leaf extract, castor oil bis-hydroxypropyl dimethicone esters, chamomilla recutita (matricaria) flower extract, ethyl menthane carboxamide, flavor, glycerin, glyceryl stearate, helianthus annuus (sunflower) seed oil, honey, lanolin, lecithin, microcrystalline wax, myristyl myristate, ozokerite, paraffin, phenoxyethanol, polybutene, purified water, sambucus nigra fruit extract, sodium ascorbyl phosphate, sodium hyaluronate, sodium saccharin, tocopheryl acetate, tridecyl neopentanoate, triethanolamine, triisononanoin
-
PRINCIPAL DISPLAY PANEL 4.25g Carton
NDC: 10157-9860-1
Blistex®
New!
EXTERNAL ANALGESIC/
LIP PROTECTANTCOLD &
ALLERGY
LIP SOOTHER™Relieves Lip Discomfort & Dryness
Due To Illness Or MedicationInfused With Vitamins C&E, Green Tea,
Elderberry, Chamomile And HoneyApplies Gently To Sore Lips
NET WT. .15 oz (4.25g)
-
INGREDIENTS AND APPEARANCE
BLISTEX COLD AND ALLERGY LIP SOOTHER
dimethicone and pramoxine hydrochloride stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10157-9860 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dimethicone (UNII: 92RU3N3Y1O) (Dimethicone - UNII:92RU3N3Y1O) Dimethicone 2 g in 100 g Pramoxine Hydrochloride (UNII: 88AYB867L5) (Pramoxine - UNII:068X84E056) Pramoxine Hydrochloride 1 g in 100 g Inactive Ingredients Ingredient Name Strength green tea leaf (UNII: W2ZU1RY8B0) chamomile (UNII: FGL3685T2X) ethyl menthane carboxamide (UNII: 6S7S02945H) glycerin (UNII: PDC6A3C0OX) glyceryl monostearate (UNII: 230OU9XXE4) sunflower oil (UNII: 3W1JG795YI) honey (UNII: Y9H1V576FH) lanolin (UNII: 7EV65EAW6H) microcrystalline wax (UNII: XOF597Q3KY) myristyl myristate (UNII: 4042ZC00DY) paraffin (UNII: I9O0E3H2ZE) phenoxyethanol (UNII: HIE492ZZ3T) water (UNII: 059QF0KO0R) european elderberry (UNII: BQY1UBX046) sodium ascorbyl phosphate (UNII: 836SJG51DR) hyaluronate sodium (UNII: YSE9PPT4TH) saccharin sodium (UNII: SB8ZUX40TY) tridecyl neopentanoate (UNII: 3Z8H1DA7J5) tangerine (UNII: KH3E3096OO) ceresin (UNII: Q1LS2UJO3A) trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10157-9860-1 1 in 1 CARTON 1 4.25 g in 1 CYLINDER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 07/01/2011 Labeler - Blistex Inc (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc 005126354 MANUFACTURE
Trademark Results [Blistex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLISTEX 88143446 5924118 Live/Registered |
Blistex Inc. 2018-10-04 |
 BLISTEX 78233453 2817472 Live/Registered |
BLISTEX INC. 2003-04-03 |
 BLISTEX 74421493 1897266 Dead/Cancelled |
Blistex Bracken Limited Partnership 1993-08-04 |
 BLISTEX 73541344 1373396 Live/Registered |
BLISTEX BRACKEN LIMITED PARTNERSHIP 1985-06-05 |
 BLISTEX 73136745 1094204 Live/Registered |
BRACKEN, JIM L. 1977-08-08 |
 BLISTEX 72226892 0804558 Live/Registered |
BLISTEX COMPANY 1965-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.