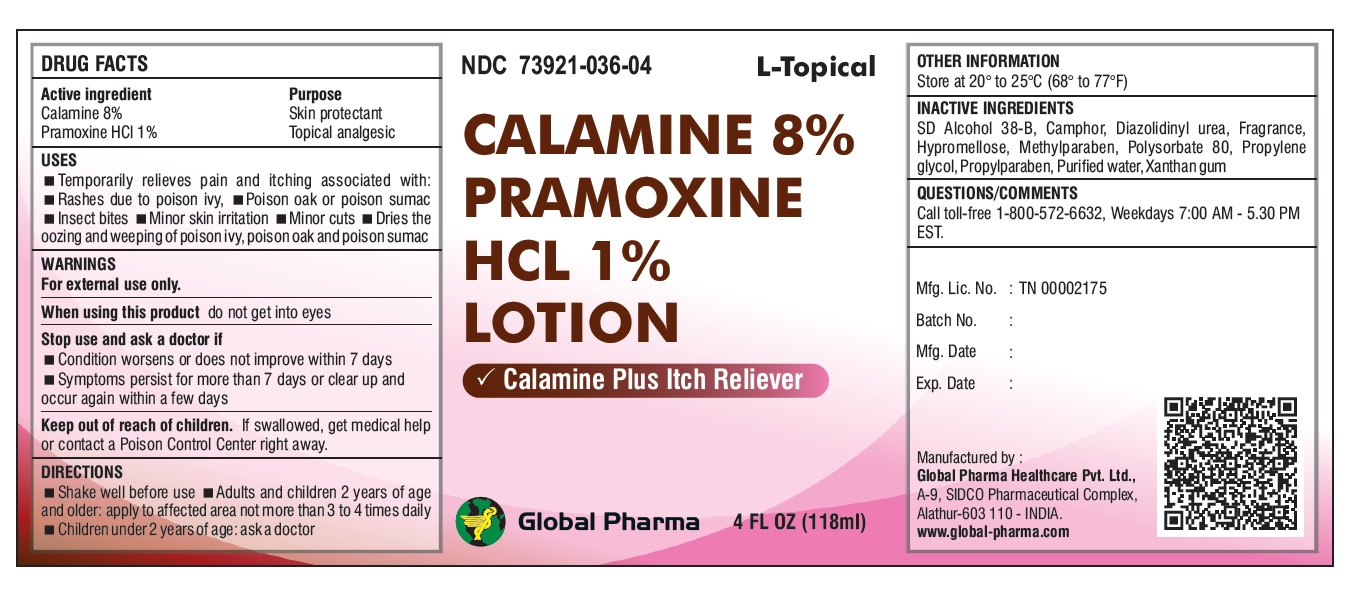
L-Topical CALAMINE 8% PRAMOXINE HCL 1% LOTION
L-Topical CALAMINE 8% PRAMOXINE HCL 1% by
Drug Labeling and Warnings
L-Topical CALAMINE 8% PRAMOXINE HCL 1% by is a Otc medication manufactured, distributed, or labeled by GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
L-TOPICAL CALAMINE 8% PRAMOXINE HCL 1%- calamine, pramoxine hydrochloride lotion
GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
L-Topical CALAMINE 8% PRAMOXINE HCL 1% LOTION
USES
Temporarily relieves pain and itching associated with:
Rashes due to poison ivy, Poison oak or poison sumac Insect bites Minor skin irritation Minor cuts Dries the oozing and weeping of poison ivy, poison oak and poison sumac
WARNINGS
For external use only.
When using this product do not get into eyes
Stop use and ask a doctor if
Condition worsens or does not improve within 7 days
Symptoms persist for more than 7 days or clear up and occur again within a few days
DIRECTIONS
Shake well before use Adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily Children under 2 years of age: ask a doctor
INACTIVE INGREDIENTS
SD Alcohol 38-B, Camphor, Diazolidinyl urea, Fragrance, Hypromellose, Methylparaben, Polysorbate 80, Propylene glycol, Propylparaben, Purified water, Xanthan gum
| L-TOPICAL CALAMINE 8% PRAMOXINE HCL 1%
calamine, pramoxine hydrochloride lotion |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED (860186917) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED | 860186917 | manufacture(73921-036) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.