Pfizer-BioNTech Vaccine
PFIZER-BIONTECH COVID-19 VACCINE by
Drug Labeling and Warnings
PFIZER-BIONTECH COVID-19 VACCINE by is a Other medication manufactured, distributed, or labeled by Pfizer Manufacturing Belgium NV, Pfizer Inc, Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC, BioNTech Manufacturing GmbH, BioNTech Manufacturing Marburg GmbH, Pfizer Ireland Pharmaceuticals, Labor LS SE & Co. KG, BioNTech Innovative Manufacturing Services GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PFIZER-BIONTECH COVID-19 VACCINE- covid-19 vaccine, mrna injection, suspension
Pfizer Manufacturing Belgium NV
----------
Pfizer-BioNTech Vaccine
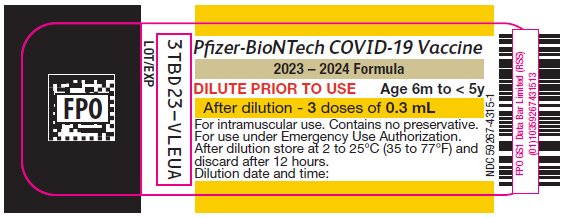
PRINCIPAL DISPLAY PANEL – 3 doses of 0.3 mL Multiple Dose Vial Label
Pfizer-BioNTech COVID-19 Vaccine
2023 – 2024 Formula
DILUTE PRIOR TO USE
Age 6m to < 5y
After dilution – 3 doses of 0.3 mL
For intramuscular use. Contains no preservative.
For use under Emergency Use Authorization.
After dilution store at 2 to 25°C (35 to 77°F) and
discard after 12 hours.
Dilution date and time:
NDC: 59267-4315-1
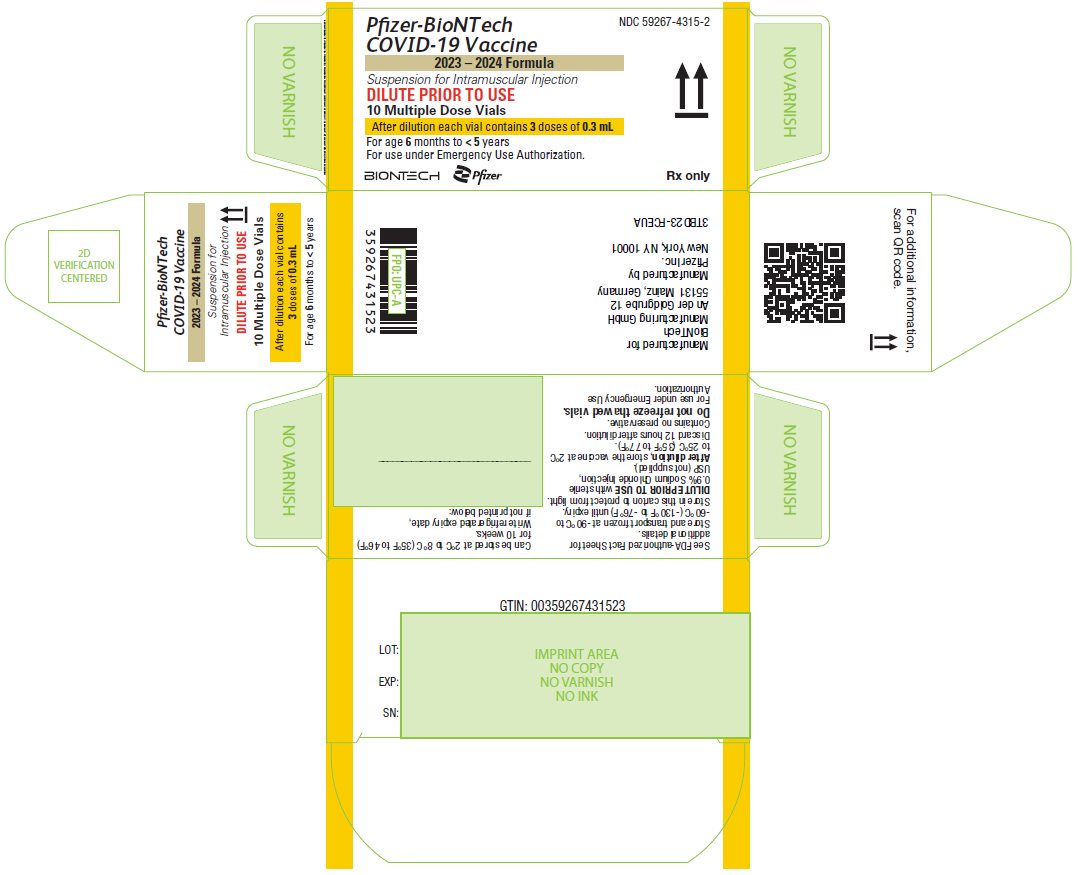
PRINCIPAL DISPLAY PANEL – 10 Multiple Dose Vial Carton
NDC: 59267-4315-2
Pfizer-BioNTech
COVID-19 Vaccine
2023 – 2024 Formula
Suspension for Intramuscular Injection
DILUTE PRIOR TO USE
10 Multiple Dose Vials
After dilution each vial contains 3 doses of 0.3 mL
For age 6 months to < 5 years
For use under Emergency Use Authorization.
BIONTECH
Pfizer
Rx only
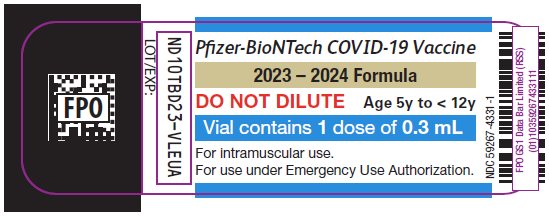
PRINCIPAL DISPLAY PANEL – 0.3 mL Single Dose Vial Label
Pfizer-BioNTech COVID-19 Vaccine
2023 – 2024 Formula
DO NOT DILUTE
Age 5y to < 12y
Vial contains 1 dose of 0.3 mL
For intramuscular use.
For use under Emergency Use Authorization.
NDC: 59267-4331-1
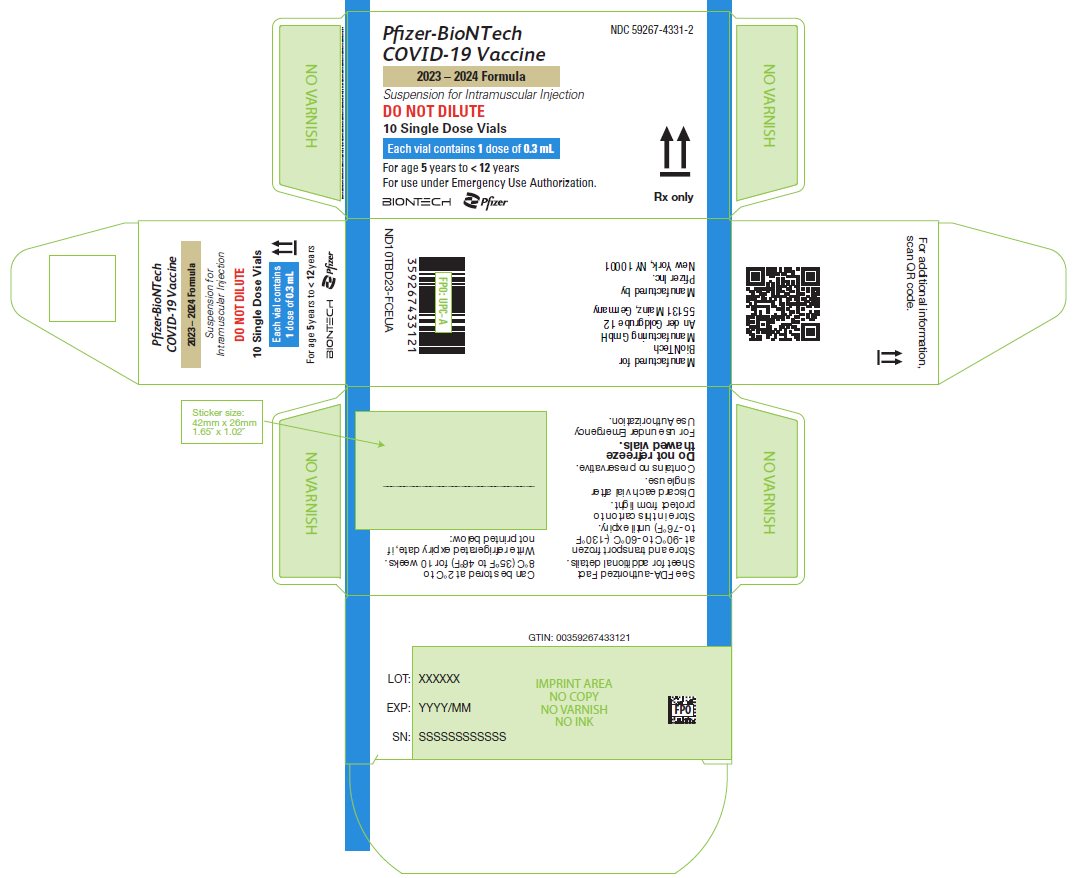
PRINCIPAL DISPLAY PANEL – 10 Single Dose Vial Carton
NDC: 59267-4331-2
Pfizer-BioNTech
COVID-19 Vaccine
2023 – 2024 Formula
Suspension for Intramuscular Injection
DO NOT DILUTE
10 Single Dose Vials
Each vial contains 1 dose of 0.3 mL
For age 5 years to < 12 years
For use under Emergency Use Authorization.
BIONTECH
Pfizer
Rx only
| PFIZER-BIONTECH COVID-19 VACCINE
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| PFIZER-BIONTECH COVID-19 VACCINE
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Manufacturing Belgium NV (370156507) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | PACK(59267-4315, 59267-4331) , MANUFACTURE(59267-4315, 59267-4331) , ANALYSIS(59267-4315, 59267-4331) , LABEL(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | 174350868 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing GmbH | 314382536 | ANALYSIS(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing Marburg GmbH | 313270335 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985586408 | ANALYSIS(59267-4315, 59267-4331) , API MANUFACTURE(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | ANALYSIS(59267-4315, 59267-4331) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Innovative Manufacturing Services GmbH | 537365801 | ANALYSIS(59267-4315, 59267-4331) | |