TULIVITE (iron- as ferrous sulfate, folic acid tablet
Tulivite by
Drug Labeling and Warnings
Tulivite by is a Other medication manufactured, distributed, or labeled by Lifsa Drugs LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HEALTH CLAIM:
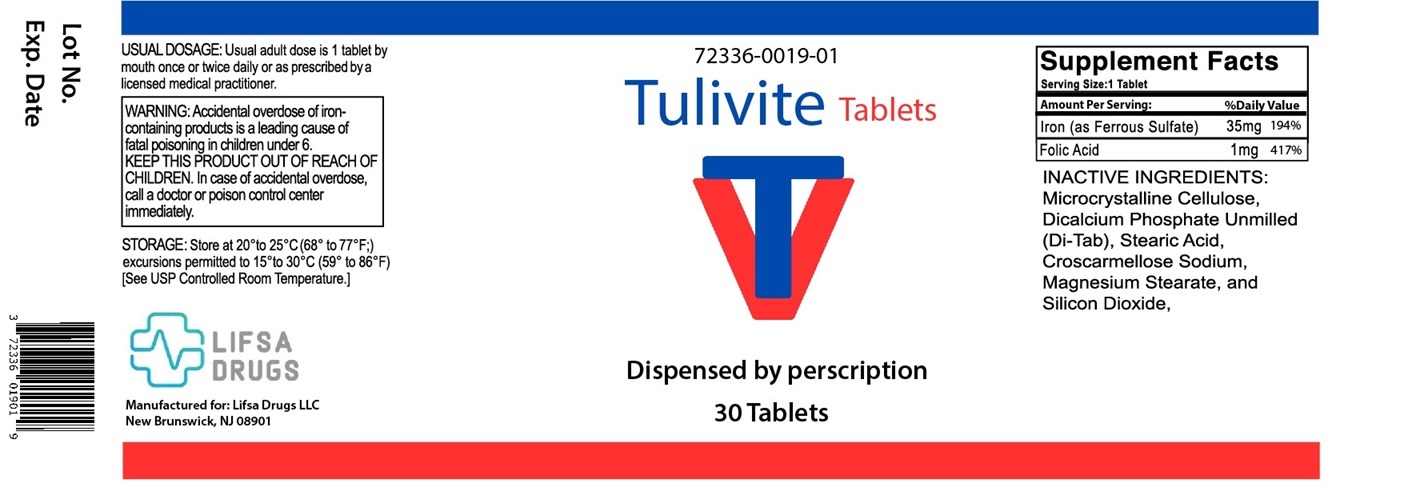
TULIVITE Tablets - Dietary Supplement
Dispensed by Prescription
Supplement Facts Serving Size: 1 Tablet
Serving per Bottle: 30Amount Per Serving: % Daily Value Iron (as Ferrous Sulfate) 35 mg 194% Folic Acid 1 mg 417% INACTIVE INGREDIENTS: Microcrystalline Cellulose, Dicalcium Phosphate Unmilled (Di-Tab), Stearic Acid, Croscarmellose Sodium, Magnesium Stearate and Silicon Dioxide.
DESCRIPTION:
TULIVITE Tablets is an orally administered prescription vitamin formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced folate supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good health is needed.
CONTRAINDICATIONS:
TULIVITE Tablets are contraindicated in patients with a known hypersensitivity to any of the ingredients. Do not take this product if you are presently taking mineral oil, unless directed by a doctor. -
WARNING:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
TULIVITE tablets should only be used under the direction and supervision of a licensed medical practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
Accidental overdose of iron-containing products a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately.Pregnancy and Lactation
TULIVITE is not intended for use in pregnant or lactating patients. -
PRECAUTIONS
PRECAUTION:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. TULIVITE Tablets should only be used under the direction and supervision of a licensed medical practitioner.
Do not take within 2 hours of taking oral tetracycline antibiotics, since oral iron products tend to interfere with absorption of tetracycline. May cause gastrointestinal discomfort, nausea, constipation or diarrhea. If you are pregnant or nursing a baby, seek advice of a health professional before using this product. U.S. Consumer Product Safety Commission requires that iron-containing medicine and vitamins with iron be packaged in child-resistant closures. Parents should always use properly re-secure safety closures.
ADVERSE REACTIONS:
Allergic sensitization has been reported following both oral and parenteral administration of folic acid. You may report side effects by calling the FDA at 1-800-FDA-1088.
- DOSAGE & ADMINISTRATION:
-
HOW SUPPLIED HEALTH CLAIM:
TULIVITE Tablets are available as white, round tablets and are available in 30-count bottles (72336-019-01*). This product is not an Orange Book product.
Dispensed by Prescription†
Manufactured for: Lifsa Drugs LLC, New Brunswick, NJ 08901
Rev. 08/23
*Lifsa Drugs LLC does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
†This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760).1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims. 1. Federal Register Notice of August 2, 1973 (38 FR 20750) 2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044) 3. Federal Register Notice of March 5, 1996 (61 FR 8760)
- STORAGE AND HANDLING:
- Packaging
-
INGREDIENTS AND APPEARANCE
TULIVITE
iron (as ferrous sulfate), folic acid tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:72336-019 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS SULFATE (UNII: 39R4TAN1VT) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 35 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72336-019-01 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/05/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 8 mm scoring 1 Labeler - Lifsa Drugs LLC (081205160)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.