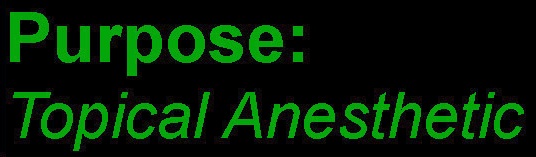SORE RELIEF- lidocaine hcl gel
sore relief by
Drug Labeling and Warnings
sore relief by is a Otc medication manufactured, distributed, or labeled by ridge properties llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SORE RELIEF
lidocaine hcl gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69804-076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 40 mg in 1000 mg Inactive Ingredients Ingredient Name Strength CASHEW OIL (UNII: PI97M87E46) 22 mg in 1000 mg BANANA (UNII: 4AJZ4765R9) 22 mg in 1000 mg NUTMEG OIL (UNII: Z1CLM48948) 133 mg in 1000 mg ACHILLEA MILLEFOLIUM OIL (UNII: 97P5D0WG43) 133 mg in 1000 mg COPAIBA OIL (UNII: 64VX45Y68N) 89 mg in 1000 mg WITCH HAZEL (UNII: 101I4J0U34) 89 mg in 1000 mg BORIC ACID (H3BO3), COMPD. WITH 2,2',2''-NITRILOTRIS(ETHANOL) (1:3) (UNII: 43O5OP619T) 58 mg in 1000 mg CARBOMER 940 (UNII: 4Q93RCW27E) 22 mg in 1000 mg BENTONITE (UNII: A3N5ZCN45C) 9 mg in 1000 mg MARANTA ARUNDINACEA ROOT (UNII: FVN346W31A) 98 mg in 1000 mg PIPER METHYSTICUM WHOLE (UNII: 3P306S300W) 285 mg in 1000 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69804-076-10 28500 mg in 1 JAR; Type 0: Not a Combination Product 11/27/2017 2 NDC: 69804-076-11 56700 mg in 1 JAR; Type 0: Not a Combination Product 11/27/2017 3 NDC: 69804-076-12 113400 mg in 1 JAR; Type 0: Not a Combination Product 11/27/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/27/2017 Labeler - ridge properties llc (029478762) Establishment Name Address ID/FEI Business Operations ridge properties llc 029478762 manufacture(69804-076)
Trademark Results [sore relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SORE RELIEF 85215657 not registered Dead/Abandoned |
Wound Care 360, Inc. 2011-01-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.