Sleep Aid by L.N.K. International, Inc. / LNK International, Inc. LNK 44-386
Sleep Aid by
Drug Labeling and Warnings
Sleep Aid by is a Otc medication manufactured, distributed, or labeled by L.N.K. International, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
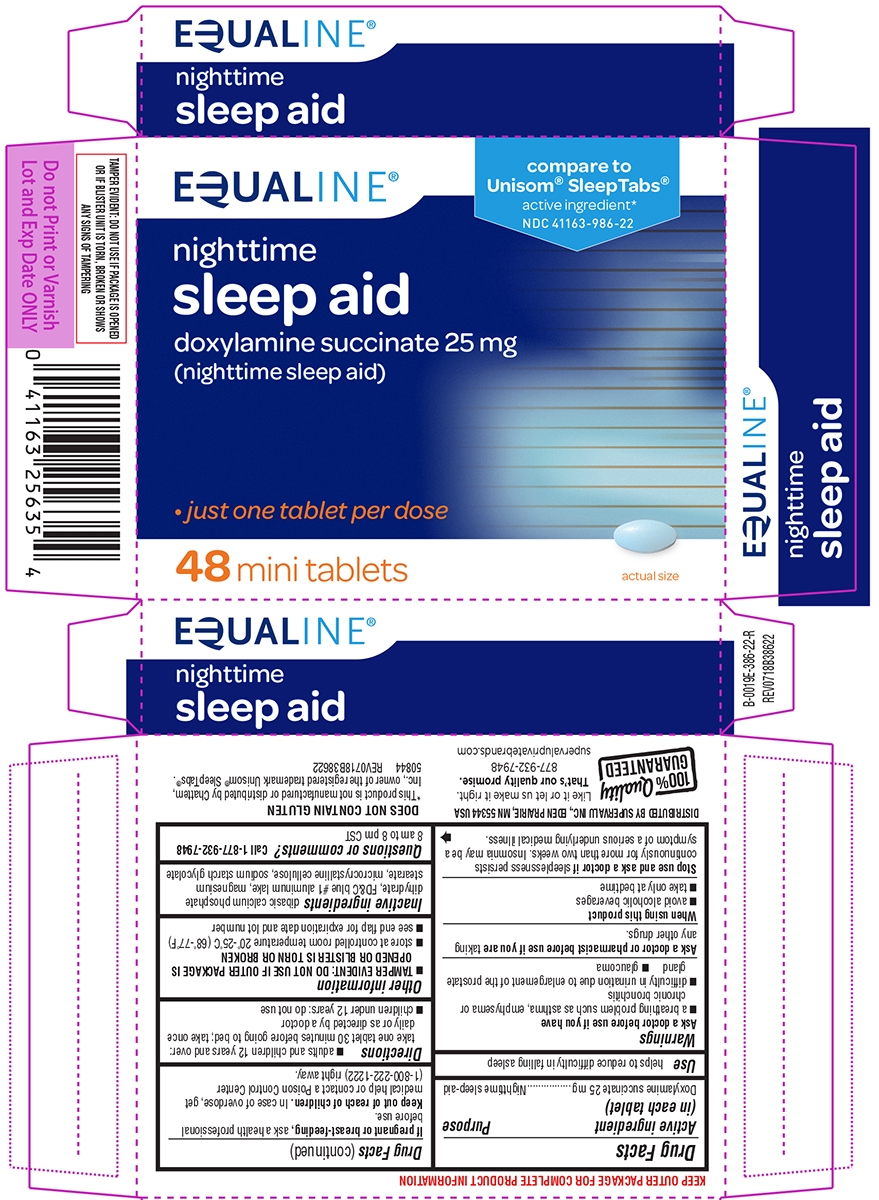
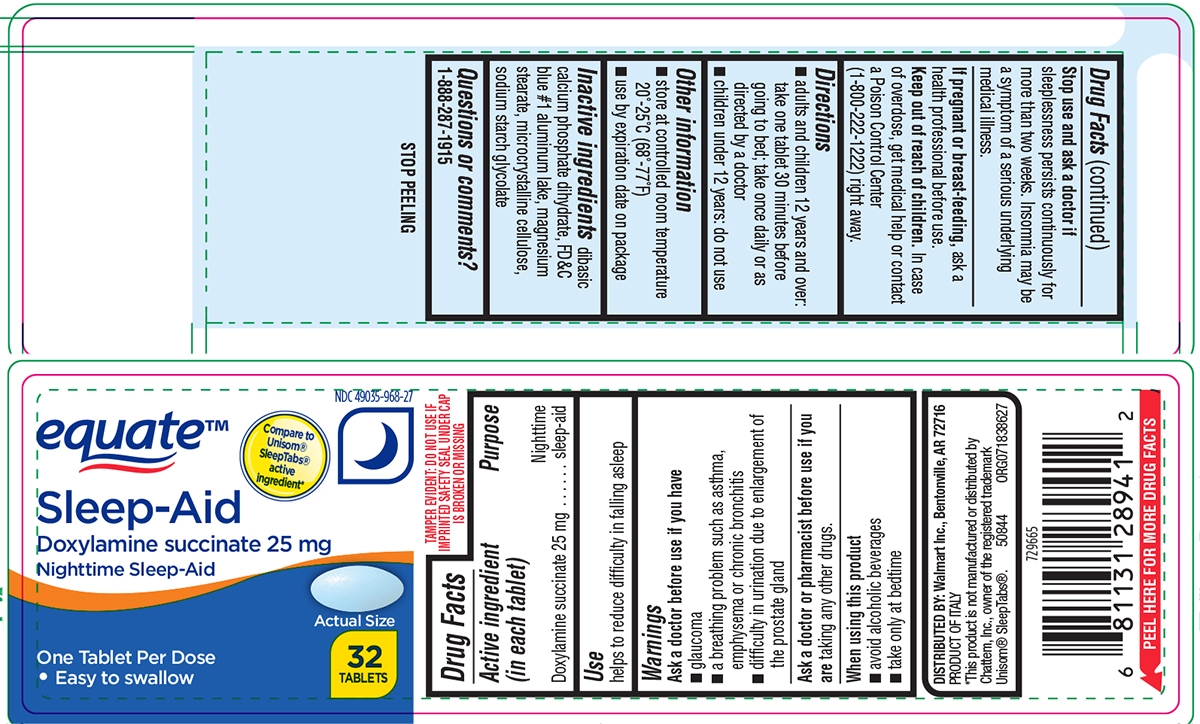
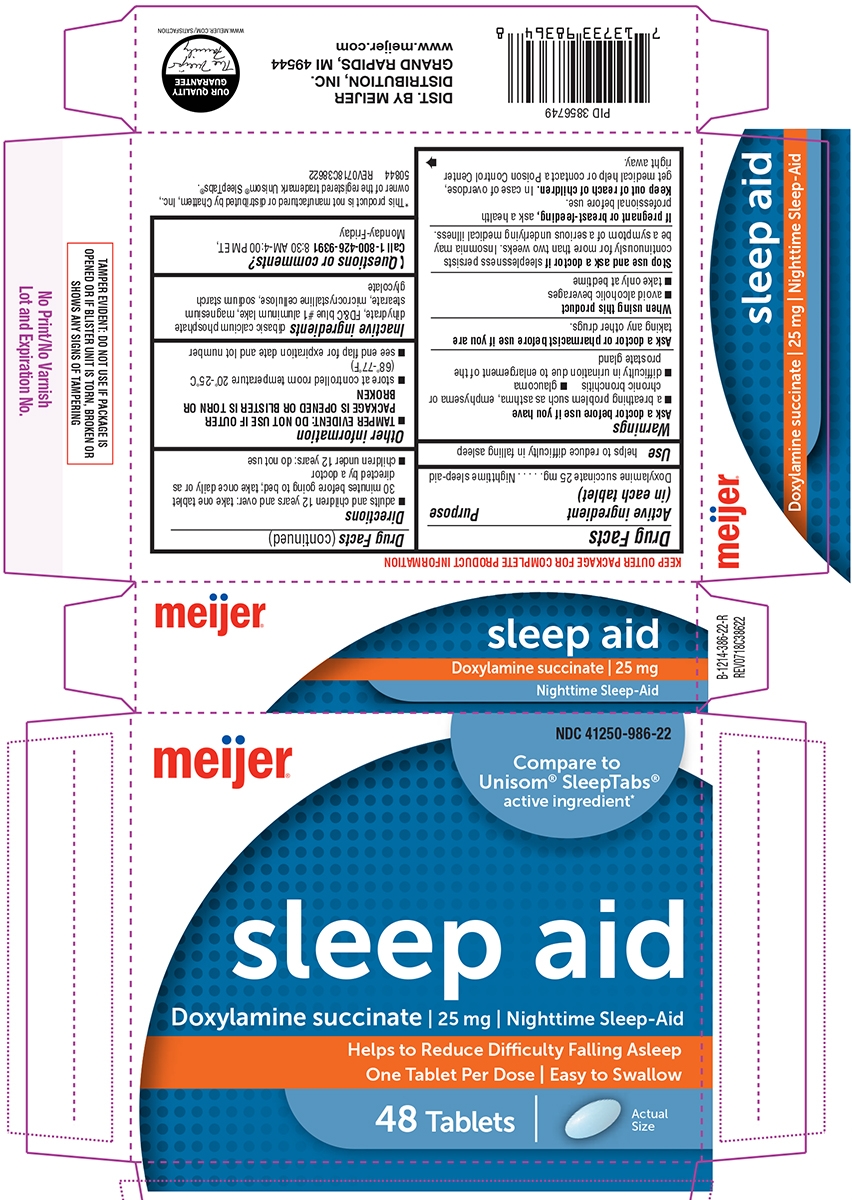
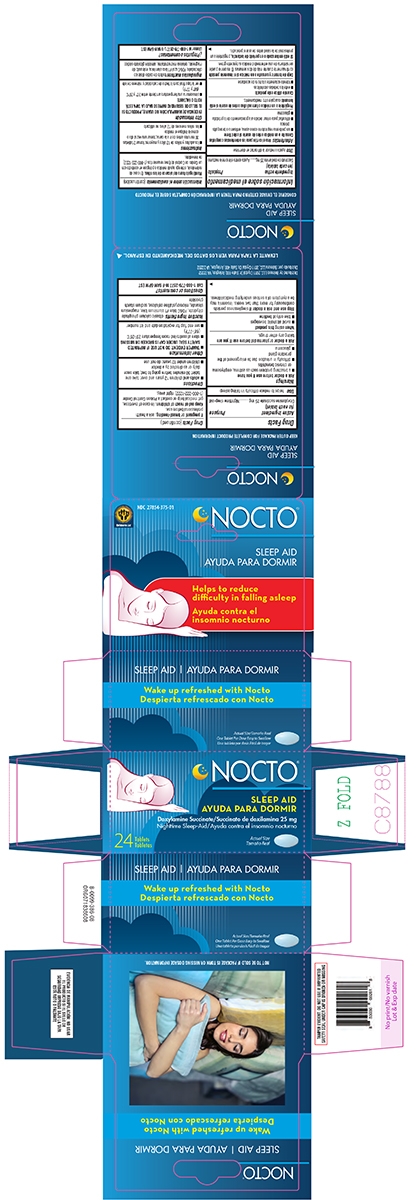
SLEEP AID- doxylamine succinate tablet
L.N.K. International, Inc.
----------
LNK 44-386
Warnings
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- glaucoma
Directions
- adults and children 12 years and over: take one tablet 30 minutes before going to bed; take once daily or as directed by a doctor
- children under 12 years: do not use
Other information
- store at controlled room temperature 20°-25°C (68°-77°F)
- see end flap for expiration date and lot number
| SLEEP AID
doxylamine succinate tablet |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(50844-952) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(50844-952) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(50844-952) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | pack(50844-952) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(50844-952) | |
Revised: 9/2021
Document Id: 09cc14a2-9eed-4d42-92a1-f0adfb912107
Set id: 5e13049d-2491-491b-a49c-de6a40ce0f1d
Version: 14
Effective Time: 20210925
Trademark Results [Sleep Aid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SLEEP AID 97858312 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-27 |
 SLEEP AID 97847521 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-20 |
 SLEEP AID 97765561 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-01-24 |
 SLEEP AID 88743606 not registered Live/Pending |
Plant Therapy LLC 2019-12-31 |
 SLEEP AID 78688138 3120450 Dead/Cancelled |
T. Harmon Inc. 2005-08-08 |
 SLEEP AID 77928191 not registered Dead/Abandoned |
EVEREST NUTRITION CORP 2010-02-04 |
 SLEEP AID 77448243 not registered Dead/Abandoned |
HEALCEUTICALS, LLC 2008-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.