Tripple Antibiotic Ointment
Bacitracin by
Drug Labeling and Warnings
Bacitracin by is a Otc medication manufactured, distributed, or labeled by Moore Medical, Sheffield Labs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BACITRACIN- antibiotic cream
Moore Medical
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
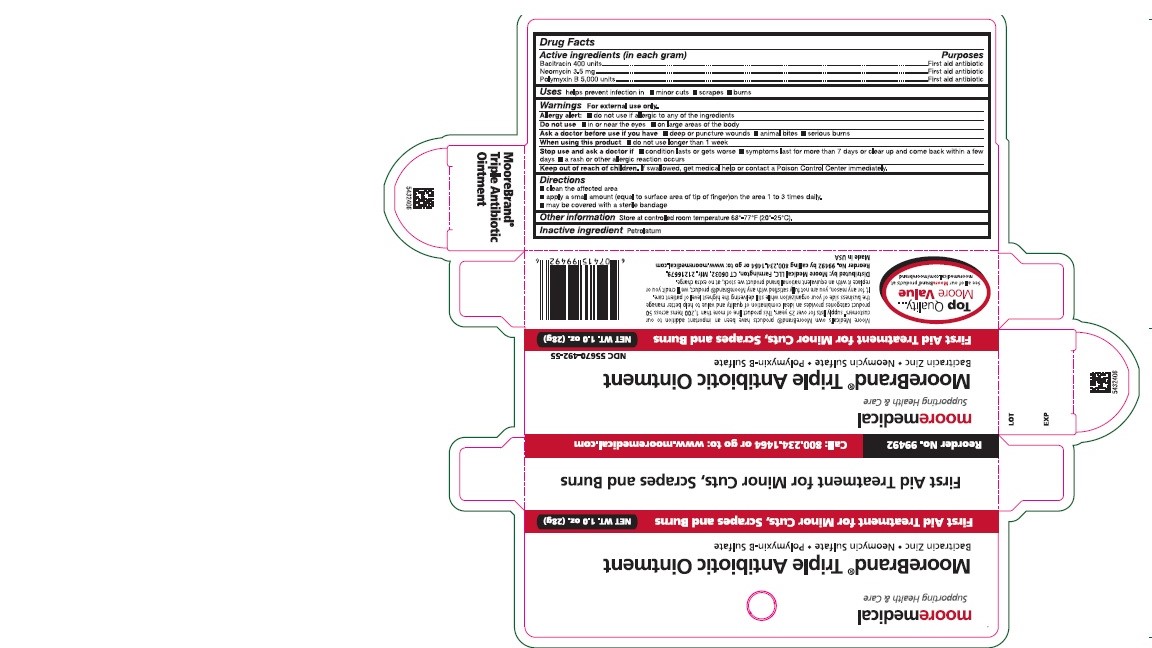
Tripple Antibiotic Ointment
Warnings
For external Use only.
Allergy Alert:
Do not use if allergic to any of the ingredients
Do not use
- In or near the eyes
- On large areas of the body
Stop use and ask a doctor if
- Condition lasts or gets worse
- Symptoms last for more than 7 days or clear up and come back within a few days
- A rash or allergic reaction occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poision Control Center immediately.
Directions
Directions
- Clean the affected area
- Apply a small amount (equal to the surface area of finger) on the area 1 to 3 times daily.
- May be covered with sterile bandage
Reorder No. 99492
Moore Medical
Supporting Health and Care
Net Wt. 1.0oz. (28g)
First aid Treatment for Minor Cuts, Scrapes and Burns
NDC: 55670-492-55
Moore Brand
Triple Antibiotic Ointment
Bacitracin Zinc, Neomycin Sulfate, Polymyxin-B Sulfate

Reorder No. 99492
Moore Medical
Supporting Health and Care
Net Wt. 1.0oz. (28g)
First aid Treatment for Minor Cuts, Scrapes and Burns
NDC: 55670-492-55
Moore Brand
Triple Antibiotic Ointment
Bacitracin Zinc, Neomycin Sulfate, Polymyxin-B Sulfate
NDC: 55670-492-55
Reorder No. 99492
UPN# 3-0607415-99492-7
Moore Medical
Supporting Health and Care
Moore Brand
Triple Antibiotic Ointment
Bacitracin Zinc, Neomycin Sulfate, Polymyxin-B Sulfate
First aid Treatment for Minor Cuts, Scrapes and Burns
1oz (28g) Tubes - 24 Tubes/Case

| BACITRACIN
antibiotic cream |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Moore Medical (051420107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sheffield Labs | 151177797 | manufacture(55670-492) | |