DIPHENHYDRAMINE HCL capsule, liquid filled
Diphenhydramine HCl by
Drug Labeling and Warnings
Diphenhydramine HCl by is a Otc medication manufactured, distributed, or labeled by AiPing Pharmaceutical, Inc., Anshi Pharmaceutical (Zhongshan) Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredient (in each softgel)
- Purpose
- Use
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- Directions
- Other Information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
Diphenhydramine Hydrochloride Softgels, USP 50 mg
Quantity : 13000 Softgels
NDC. No : 11788-018-00WARNING:
KEEP OUT OF THE REACH OF CHILDREN. THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY. CONTENTS SHOULD BE REPACKAGED IMMEDIATELY AND LABELED IN STRICK CONFORMANCE WITH THE FOOD DRUG & COSMETIC ACT AND REGULATIONS THEREUNDER.
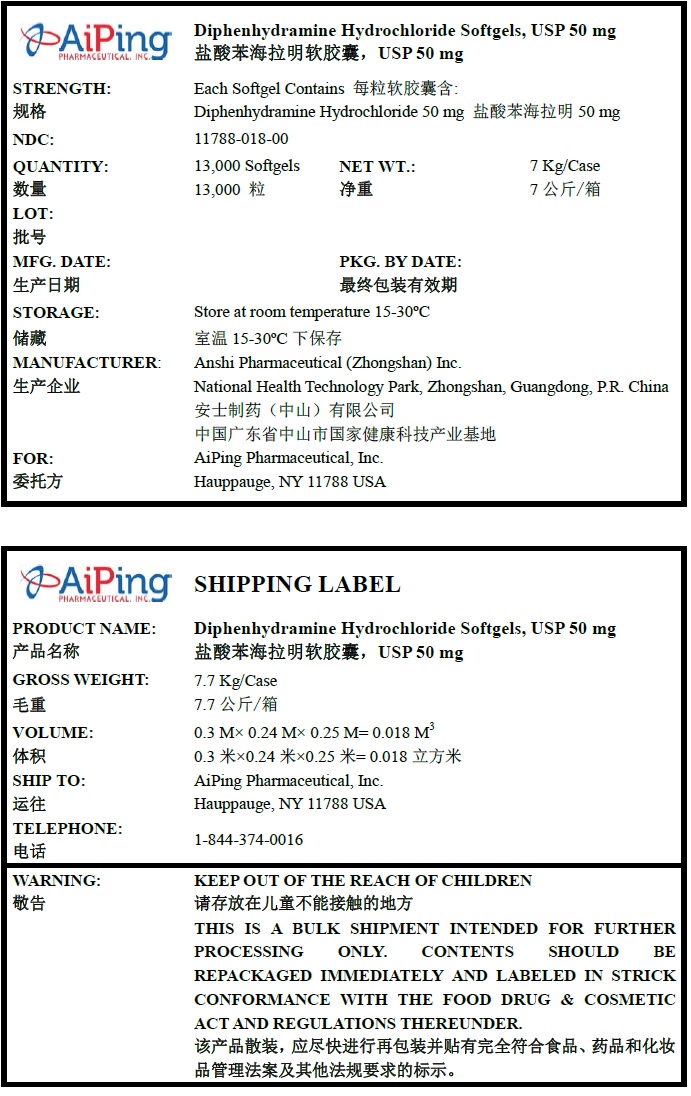
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL
diphenhydramine hcl capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11788-018 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) Product Characteristics Color blue Score no score Shape CAPSULE Size 14mm Flavor Imprint Code AP018 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11788-018-00 1 in 1 BOX 01/31/2015 1 13000 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/31/2015 Labeler - AiPing Pharmaceutical, Inc. (079674526) Registrant - AiPing Pharmaceutical, Inc. (079674526) Establishment Name Address ID/FEI Business Operations Anshi Pharmaceutical (Zhongshan) Inc. 528101821 manufacture(11788-018)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.