BIO SPECTRA ATTITUDE ®
BIO SPECTRA by
Drug Labeling and Warnings
BIO SPECTRA by is a Otc medication manufactured, distributed, or labeled by BIO SPECTRA, Federal Package Network Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
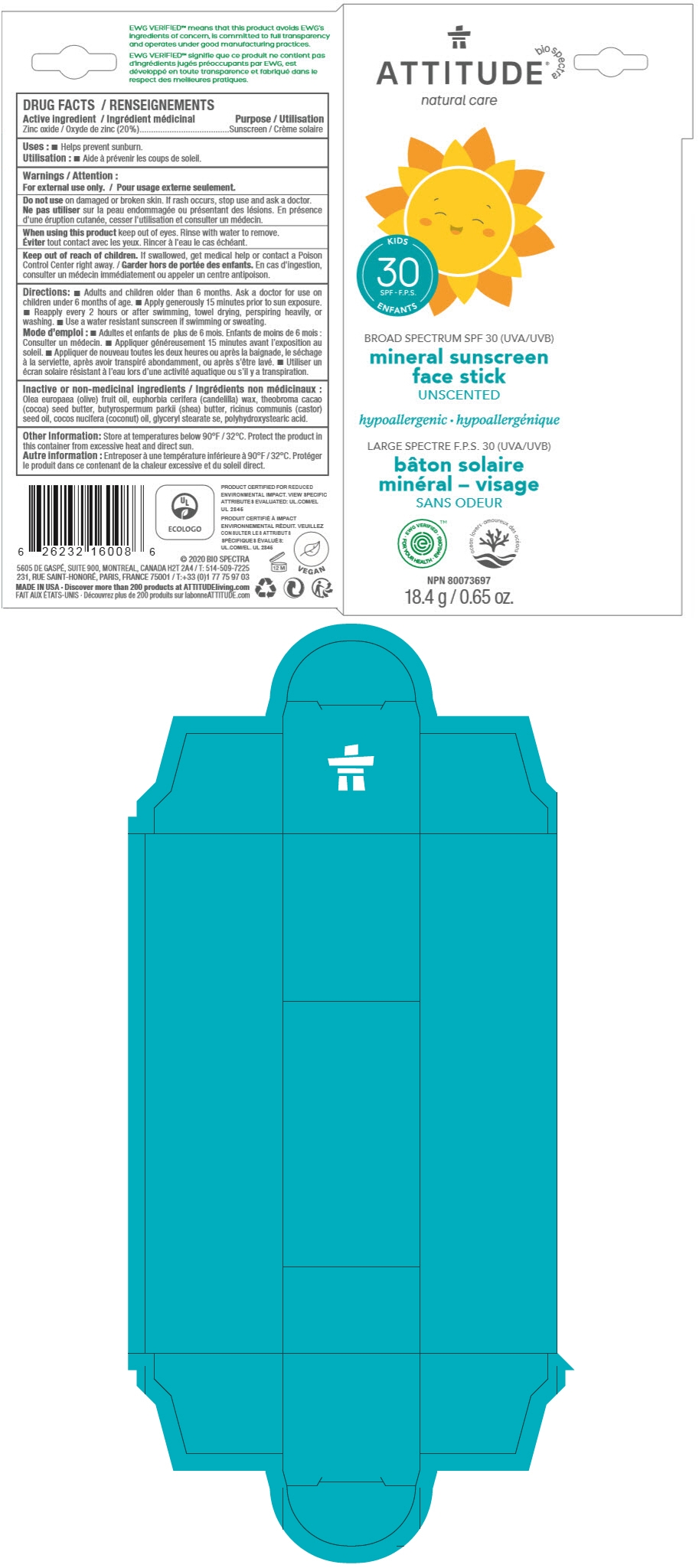
BIO SPECTRA ATTITUDE- zinc oxide stick
BIO SPECTRA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BIO SPECTRA
ATTITUDE
®
Directions
- Adults and children older than 6 months. Ask a doctor for use on children under 6 months of age.
- Apply generously 15 minutes prior to sun exposure.
- Reapply every 2 hours or after swimming, towel drying, perspiring heavily, or washing.
- Use a water resistant sunscreen if swimming or sweating.
Inactive or non-medicinal ingredients
Olea europaea (olive) fruit oil, euphorbia cerifera (candelilla) wax, theobroma cacao (cocoa) seed butter, butyrospermum parkii (shea) butter, ricinus communis (castor) seed oil, cocos nucifera (coconut) oil, glyceryl stearate se, polyhydroxystearic acid.
| BIO SPECTRA
ATTITUDE
zinc oxide stick |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - BIO SPECTRA (201137051) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Federal Package Network Inc. | 118758804 | manufacture(61649-900) | |
Revised: 7/2023
Document Id: 00239ce6-f65a-be02-e063-6294a90a259f
Set id: 5e815d7e-95bf-41da-bcc3-d8b21470fe05
Version: 2
Effective Time: 20230710
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.