VIMKUNYA- chikungunya vaccine, recombinant injection, suspension
VIMKUNYA by
Drug Labeling and Warnings
VIMKUNYA by is a Other medication manufactured, distributed, or labeled by Bavarian Nordic A/S, Bavarian Nordic Berna GmbH, Grand River Aseptic Manufacturing Inc, Grand River Aseptic Manufacturing Inc., Sharp Packaging Services, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIMKUNYATM safely and effectively. See full prescribing information for VIMKUNYATM.
VIMKUNYA™ (Chikungunya Vaccine, Recombinant) injectable suspension, for intramuscular use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
VIMKUNYA is a vaccine indicated for the prevention of disease caused by chikungunya virus in individuals 12 years of age and older. (1)
The indication is approved under accelerated approval based on anti-chikungunya virus neutralizing antibody levels. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
VIMKUNYA is an injectable suspension. A single dose is 0.8 mL. (3)
CONTRAINDICATIONS
Do not administer VIMKUNYA to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of VIMKUNYA. (4)
WARNINGS AND PRECAUTIONS
- Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of VIMKUNYA. (5.1)
- Immunocompromised individuals, including individuals receiving immunosuppressive therapy, may have a diminished immune response to VIMKUNYA. (5.2)
- Syncope (fainting) may occur in association with administration of injectable vaccines including VIMKUNYA. Procedures should be in place to avoid injury from fainting. (5.3)
ADVERSE REACTIONS
- The most commonly reported solicited adverse reactions (>10%) in individuals 12 through 64 years of age were injection site pain (23.7%), fatigue (19.9%), headache (18.0%), and myalgia (17.6%). (6.1)
- The most commonly reported solicited adverse reactions (>5%) in individuals 65 years of age and older were injection site pain (5.4%), myalgia (6.3%), and fatigue (6.3%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic Inc. at 1-833-365-9596 or drug.safety@bavarian-nordic.com or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
5.2 Altered Immunocompetence
5.3 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VIMKUNYA is a vaccine indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 12 years of age and older.
This indication is approved under accelerated approval based on anti-CHIKV neutralizing antibody levels [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use.
2.2 Preparation
Shake the pre-filled syringe vigorously immediately before use to form a homogeneous suspension. After shaking, the suspension should be a white, cloudy liquid.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer VIMKUNYA to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of VIMKUNYA.
-
6 ADVERSE REACTIONS
In participants 12 through 64 years of age who received VIMKUNYA, the most common solicited local adverse reaction (>10%) was injection site pain (23.7%). The most common solicited systemic adverse reactions (>10%) were fatigue (19.9%), headache (18.0%), and myalgia (17.6%).
In participants 65 years of age and older who received VIMKUNYA, the most common solicited local adverse reaction (>5%) was injection site pain (5.4%). The most common solicited systemic adverse reactions (>5%) were myalgia (6.3%) and fatigue (6.3%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of VIMKUNYA was evaluated in two clinical trials [Study 1 (NCT05072080) and Study 2 (NCT05349617)], both conducted in the United States, in which a total of 3,667 participants 12 years of age and older received a single dose of VIMKUNYA or placebo [formulation buffer containing 218 mM sucrose, 10 mM potassium phosphate, 25 mM sodium citrate, pH 7.0 (see Description (11)].
Study 1 was a multicenter, randomized, placebo-controlled, double-blinded trial. Individuals aged 12 through 64 years were randomized in a 6:1 ratio, stratified by age stratum (12-17, 18-45, and 46-64 years of age), to receive a single dose of VIMKUNYA (n=2,794) or placebo (n=464).
Among the overall 3,258 participants randomized in Study 1, the median age was 38 years [with 254 (8%) participants 12 through 17 years of age, 1906 (58%) participants 18 through 45 years of age, and 1098 (34%) participants 46 through 64 years of age]; 51.2% were female; 73.2% were White, 19.1% Black or African American, 2.9% Asian, 1.0% American Indian or Alaska Native, 0.3% Native Hawaiian or Pacific Islander, 2.6% multiple racial groups; and 17.7% were of Hispanic or Latino ethnicity.
Study 2 was a multicenter, randomized, placebo-controlled, double-blinded trial. Individuals aged 65 years and older were randomized in a 1:1 ratio, stratified by age stratum (65-74 and ≥75 years of age), to receive a single dose of VIMKUNYA (n=206) or placebo (n=207).
Among the overall 413 participants enrolled in Study 2, the median age was 70 years, and 58.6% were female; 83.3% were White, 11.9% Black or African American, 1.2% Asian, 0.5% American Indian or Alaska Native, 2.2% multiple racial groups; and 44.3% were of Hispanic or Latino ethnicity.
In Studies 1 and 2, solicited adverse reactions were collected via electronic diary from the vaccination day through 7 days post-vaccination (an 8-day period). Unsolicited adverse events were monitored for 28 days post-vaccination. Serious adverse events were monitored through 6 months post-vaccination. New onset or worsening arthralgia that was medically attended was monitored through 6 months post-vaccination.
Solicited Adverse Reactions
The percentage of participants in Study 1 reporting solicited local (injection site) and systemic adverse reactions is shown in Table 1. In Study 1, the median day of onset was Day 1 for local reactions (Day 1 was the day of vaccination) and Day 2 for systemic reactions following administration of VIMKUNYA. Local and systemic adverse reactions resolved with a median duration of 1 day.
Table 1. Percentages of Participants with Solicited Local and Systemic Adverse Reactions Through 7 Days After Vaccination (Study 1a, 12 through 64 years of age)
Note: Solicited adverse reactions were collected from the vaccination day through 7 days post-vaccination (an 8-day period). Percentages are based on the number of participants in the Study 1 safety population with at least one diary observation for a given symptom for a given day.
a NCT05072080
b Severity=mild, moderate, severe intensity. Absence of rows for severe reactions indicates that no reactions of this severity were reported in either group.
c Defined as mild (no interference with activity), moderate (repeated use of non-narcotic pain reliever >24 hours or interference with activity), severe (any use of narcotic pain reliever or prevents daily activity).
d Defined as mild (no interference with activity), moderate (some interference with activity), severe (prevents daily activity).
e Defined as mild (no interference with activity or 1 – 2 episodes/24 hours), moderate (some interference with activity or >2 episodes/24 hours), severe (prevents daily activity, requires outpatient intravenous hydration).
f The denominator for injection site pain, redness, swelling, arthralgia, chills, fatigue, myalgia, and nausea is 2,764 for VIMKUNYA and 458 for placebo.
g The denominator for fever is 2,760 for VIMKUNYA and 457 for placebo.
h The denominator for headache is 2,765 for VIMKUNYA and 458 for placebo.Adverse Reaction
VIMKUNYA
N=2790
%Placebo
N=464
%Solicited Local (Injection Site)
Adverse ReactionsbPain (any)c,f
23.7
10.7
Pain (severe)
0.1
0
Redness/Erythema (≥25 mm)f
0.5
0
Redness/Erythema (>100 mm)
<0.1
0
Swelling (≥25 mm)f
0.4
0
Solicited Systemic Adverse Reactionsb
Fatigue (any)d,f
19.9
17.0
Fatigue (severe)
0.7
0.2
Headache (any)c,h
18.0
16.6
Headache (severe)
0.3
0.4
Myalgia/Muscle Pain (any)d,f
17.6
9.6
Myalgia/Muscle Pain (severe)
0.4
0.4
Chills (any)d,f
8.6
3.3
Chills (severe)
0.1
0
Arthralgia/Joint Pain (any)d,f
7.7
7.2
Arthralgia/Joint Pain (severe)
0.3
0.2
Nausea (any)e,f
7.5
6.6
Nausea (severe)
0.4
0
Fever (≥38.0°C or ≥100.4°F)g
0.9
0.2
Fever (≥39.0°C or ≥102.1°F)
0.2
0
In Study 1, solicited adverse reactions were reported by 94 (44.1%) participants12 through 17 years of age, 676 (41.8%) participants 18 through 45 years of age, and 289 (31.0%) participants 46 through 64 years of age in the VIMKUNYA group.
The percentage of participants in Study 2 reporting solicited local and systemic adverse reactions is shown in Table 2. In Study 2, the median day of onset was Day 2 for both local and systemic reactions (Day 1 was the day of vaccination) following administration of VIMKUNYA. Local adverse reactions resolved with a median duration of 1 day and systemic adverse reactions resolved with a median duration of 2 days.
Table 2. Percentages of Participants with Solicited Local and Systemic Adverse Reactions Through 7 Days After Vaccination (Study 2a, 65 years of age and greater)
Note: Solicited adverse reactions were collected from the vaccination day through 7 days post-vaccination (an 8-day period). N=Number of participants in the Study 2 safety population with at least one diary observation for a given symptom for a given day.
a NCT05349617
b Severity=mild, moderate, severe intensity. Absence of rows for severe reactions indicates that no reactions of this severity were reported in either group.
c Defined as mild (no interference with activity), moderate (repeated use of non-narcotic pain reliever > 24 hours or interference with activity), severe (any use of narcotic pain reliever or prevents daily activity).
d Defined as mild (no interference with activity), moderate (some interference with activity), severe (prevents daily activity).
e Defined as mild (no interference with activity or 1 – 2 episodes/24 hours), moderate (some interference with activity or >2 episodes/24 hours), severe (prevents daily activity, requires outpatient intravenous hydration).Adverse Reaction
VIMKUNYA
N=205
%Placebo
N=200
%Solicited Local (Injection Site)
Adverse ReactionsbPain (any)c
5.4
1.5
Redness/Erythema (≥25 mm)
0
0.5
Swelling (≥25 mm)
0
0
Solicited Systemic Adverse Reactionsb
Myalgia/Muscle Pain (any)d
6.3
6.5
Fatigue (any)d
6.3
6.0
Fatigue (severe)
0.5
0
Headache (any)c
4.4
7.5
Headache (severe)
0.5
0
Arthralgia/Joint Pain (any)d
2.9
4.0
Chills (any)d
2.9
3.0
Nausea (any)e
2.9
1.5
Fever (≥38.0°C or ≥100.4°F)
0
1.0
Unsolicited Adverse Events
In Study 1, unsolicited adverse events that occurred within 28 days following vaccination were reported in 15.5% of 2,790 participants who received VIMKUNYA and 12.7% of 464 participants who received placebo. There was one report of severe dehydration considered related to VIMKUNYA.
In Study 2, unsolicited adverse events that occurred within 28 days following vaccination were reported in 12.6% of 206 participants who received VIMKUNYA and 15.5% of 207 participants who received placebo. There were no severe unsolicited adverse events considered related to VIMKUNYA.
Medically Attended New Onset or Worsening Arthralgia
In Study 1, 3 (0.1%) participants in the VIMKUNYA group (n=2,790) and 1 (0.2%) participant in the placebo group (n=464) reported new onset or worsening arthralgia that was medically attended and considered possibly or probably related to VIMKUNYA (beginning 2, 14 and 84 days post-vaccination, respectively) or possibly related to placebo (beginning 1 day post-vaccination); none of these reactions were reported as serious or severe (defined as those that prevented daily activity and/or required medical intervention).
In Study 2, there were no participants in the VIMKUNYA or placebo groups with new onset or worsening arthralgia that was medically attended and considered at least possibly related to the study intervention.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to VIMKUNYA during pregnancy. Women who receive VIMKUNYA during pregnancy are encouraged to contact, or have their healthcare provider contact, 1-888-230-2491 to enroll in or obtain information about the registry.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no clinical studies of VIMKUNYA in pregnant women. Data on VIMKUNYA administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study was performed in female rabbits, administered the equivalent of a single human dose of VIMKUNYA on 5 occasions, twice prior to mating, twice during gestation and once during lactation. In this study, postnatal survival of the kits was reduced; there were no adverse effects on other postnatal development parameters. There were no adverse effects on female fertility; and there was no evidence of harm to the fetus due to the vaccine. A developmental toxicity study was performed in female rats administered the equivalent of a single human dose of VIMKUNYA on 5 occasions, twice prior to mating, twice during gestation and once during lactation. In this study there were no adverse effects on postnatal survival and on other postnatal development parameters. There were no adverse effects on female fertility. [see Data].
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Vertical transmission of wild-type CHIKV to neonates from pregnant women with viremia at delivery is common and can cause severe, potentially fatal CHIKV disease in neonates, with neurologic (e.g., encephalopathy, intracranial hemorrhage) and myocardial manifestations.1
Animal Data
In a pre- and postnatal developmental study with an embryo-fetal development toxicity phase performed in female rabbits, a full human dose (0.8 mL) of VIMKUNYA was administered by intramuscular injection on five occasions: 28 and 14 days prior to start of cohabitation, on Gestation Days 7 and 21 and on Lactation Day 7. Among kits born in the control group, 69% of the kits [95% confidence interval (CI) (57.8%, 80.1%)] survived compared to the 42% of kits [95% CI (31.5%, 52.8%)] born to vaccinated mothers (the historical control data showed a range for postnatal survival from 47.6% to 91.4% with a mean of 71%, from 18 studies); other postnatal development parameters were not affected. There were no adverse effects on female fertility; and there was no evidence of harm to the fetus due to the vaccine.
In a pre- and postnatal developmental study performed in female rats, a full human dose (0.8 mL) of VIMKUNYA was administered by intramuscular injection on five occasions: 28 and 14 days prior to start of cohabitation, on Gestation Days 7 and 21 and on Lactation Day 7. No vaccine related adverse effects on female fertility or postnatal development were observed.
8.2 Lactation
Risk Summary
Human data are not available to assess the impact of VIMKUNYA on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VIMKUNYA and any potential adverse effects on the breastfed child from VIMKUNYA or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of VIMKUNYA in individuals 12 through 17 years of age are based on data from this age group and data from adults [see Adverse Reactions (6.1) and Clinical Studies (14)].
The safety and effectiveness of VIMKUNYA in individuals younger than 12 years of age have not been established.
8.5 Geriatric Use
In Study 2, the 206 individuals who received VIMKUNYA were 65 years of age and older; and among these, 47 individuals (22.8%) were 75 years of age and older. The incidence of solicited adverse reactions in individuals 65 years of age and older was generally lower than that observed in individuals less than 65 years of age [see Adverse Reactions (6.1)]. The seroresponse rate in individuals 65 years of age and older was lower than that observed in individuals less than 65 years of age [see Clinical Studies (14)].
-
11 DESCRIPTION
VIMKUNYA, Chikungunya Vaccine, Recombinant, is a sterile injectable suspension for intramuscular use. VIMKUNYA contains purified virus-like particles (VLPs) consisting of CHIKV capsid protein (C) and envelope proteins E1 and E2, derived from CHIKV Senegal strain 37997. The VLPs are produced by transfecting an expression plasmid that encodes for the CHIKV structural polyprotein C-E3-E2-6K-E1 in HEK293 (a continuous line of human embryonic kidney cells) in media containing amino acids, vitamins, and minerals. The VLPs containing C, E1 and E2, are harvested from the media and then purified by a series of chemical and physical methods. After sterile filtration, the purified VLPs are mixed with formulation buffer and adsorbed on aluminum hydroxide [Al(OH)3] as adjuvant.
Each 0.8-mL dose contains approximately 40 mcg of CHIKV VLPs. Each dose also contains 867 mcg Al(OH)3 (approximately 300 mcg aluminum), 59.7 mg sucrose, 5.9 mg sodium citrate dihydrate, 0.9 mg potassium phosphate dibasic, 0.4 mg potassium phosphate monobasic, and water for injection. Each dose may contain residual amounts of HEK293 cell protein (less than 400 ng/dose), HEK293 cell DNA (less than 10 ng/dose), poloxamer 188 (less than 2850 mcg/dose), polyethyleneimine (less than 4 mcg/dose), valproic acid (less than 12.8 mcg/dose), Benzonase (less than 1.6 ng/dose), and plasmid DNA (less than 3.6 ng/dose), from the manufacturing process.
VIMKUNYA does not contain a preservative or antibiotics.
The syringe stoppers and caps are not made with natural rubber latex.
- 12 CLINICAL PHARMACOLOGY
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
VIMKUNYA has not been evaluated for the potential to cause carcinogenicity, mutagenic potential, or for impairment of male fertility in animals.
13.2 Animal Toxicology and/or Pharmacology
A passive transfer study was performed in non-human primates (NHPs) using human anti-CHIKV immune sera collected from two Phase 2 studies (NCT03483961 and NCT03992872), from participants who received a single dose of a vaccine formulation containing the same CHIKV VLP used in VIMKUNYA. Sera obtained on Day 22 after vaccination were pooled to generate a serum pool with a NT80 titer of 2470, as determined by a CHIKV luciferase neutralization assay. In the passive transfer study, 20 CHIKV-naïve cynomolgus macaques (M. fascicularis) were administered human anti-CHIKV immune sera at four dose levels (2.4, 1.2, 0.6 and 0.3 mL/kg) and 6 CHIKV-naïve cynomolgus macaques were administered non-immune control sera by intravenous injection. One day after the transfers, serum samples were obtained from the macaques to determine pre-challenge anti-CHIKV neutralizing antibody titers by the CHIKV luciferase neutralization assay. On the same day following sera collection, animals were challenged via the subcutaneous route with 100,000 Plaque Forming Units of wild-type CHIKV strain La Réunion 2006-OPY1, corresponding to 1000 times the 50% animal infectious dose. Animal monitoring included assessment of wild-type CHIKV-induced viremia by plaque assay and RT-qPCR through 10 days after challenge. For those NHPs that received anti-CHIKV sera, no infectious virus was detected in the blood, and the amounts of CHIKV RNA in the blood were reduced in a dose-dependent manner compared with NHPs who received non-immune human sera. Data from the NHP study were analyzed by logistic regression and a NT80 titer of ≥100 was determined to be reasonably likely to predict clinical benefit in the Phase 3 studies.
-
14 CLINICAL STUDIES
The immunogenicity of VIMKUNYA was assessed in Studies 1 and 2 [see Adverse Reactions (6.1)]. In both studies, the assessment of effectiveness was based on seroresponse rate at Day 22 and the serum neutralizing antibody (SNA) geometric mean titer (GMT) at Day 22. The seroresponse rate and GMT 21 days after a single dose of VIMKUNYA in Study 1 is presented in Table 3.
Table 3. GMTs and Seroresponse Rates 21 Days Post-vaccination as Determined by Anti-CHIKV Human SNA Assay in Study 1 (12 through 64 years of age)
CHIKV=Chikungunya virus; CI=confidence interval; GMT=geometric mean titer; SNA=serum neutralizing antibody.
N=Exposed participants who had at least one post-injection anti-CHIKV SNA result, no measurable anti-CHIKV SNA at Day 1, an evaluable Day 22 serum sample result within window (Day 19 through Day 27, inclusive), and no important protocol deviation deemed exclusionary.
a GMT estimates, together with their 95% CIs, are derived from an ANOVA model that includes site and vaccine group as fixed effects, assuming normality of the log titers. GMTs ratios and 95% CIs are derived from the same model.
b 95% CIs of seroresponse rates are based on the Wilson method.
c 95% CIs of seroresponse rate differences are based on the Newcombe hybrid score method.
d Seroresponse rate is defined as the percentage of participants who have achieved an anti-CHIKV neutralizing antibody titer ≥100 measured by luciferase-based CHIKV neutralization assay. The neutralizing antibody titer is expressed as a serum dilution achieving 80% neutralization (NT80). This threshold was derived from a non-human primate model [see Nonclinical Toxicology (13.2)].
e Success criterion: lower bound of the 95% CI for GMT ratio > 1.
f Success criterion: lower bound of the 95% CI for seroresponse rate difference ≥70%.GMTa
VIMKUNYA
N=2559
(95% CI)GMTa
Placebo
N=424
(95% CI)GMT Ratioa,e,
VIMKUNYA
over Placebo
(95% CI)1597.0
(1504.1, 1695.6)7.9
(7.0, 8.8)203.3
(181.1, 228.2)Seroresponse
Rateb,d
VIMKUNYA
N=2559
% (95% CI)Seroresponse
Rateb,d
Placebo
N=424
% (95% CI)Seroresponse Rate
Differencec,f
VIMKUNYA
minus Placebo
(95% CI)97.8
(97.2, 98.3)1.2
(0.5, 2.7)96.6
(95.0, 97.5)The seroresponse rate and GMT 21 days after a single dose of VIMKUNYA in Study 2 is presented in Table 4.
Table 4. GMTs and Seroresponse Rates 21 Days Post-vaccination as Determined by Anti-CHIKV Human SNA Assay in Study 2 (65 years of age and older)
CHIKV=Chikungunya virus; CI=confidence interval; GMT=geometric mean titer; SNA=serum neutralizing antibody.
N=Exposed participants who had at least one post-injection anti-CHIKV SNA result, no measurable anti-CHIKV SNA at Day 1, an evaluable Day 22 serum sample result within window (Day 19 through Day 27, inclusive), and no important protocol deviation deemed exclusionary.
a GMT estimates, together with their 95% CIs, are derived from an ANOVA model that includes site and vaccine group as fixed effects, assuming normality of the log titers. GMTs ratios and 95% CIs are derived from the same model.
b 95% CIs of seroresponse rates are based on the Wilson method.
c 95% CIs of seroresponse rate differences are based on the Newcombe hybrid score method.
d Seroresponse rate is defined as the percentage of participants who have achieved an anti-CHIKV neutralizing antibody titer ≥100 measured by luciferase-based CHIKV neutralization assay. The neutralizing antibody titer is expressed as a serum dilution achieving 80% neutralization (NT80). This threshold was derived from a non-human primate model [see Nonclinical Toxicology (13.2)].
e Success criterion: lower bound of the 95% CI for GMT ratio > 1.
f Success criterion: lower bound of the 95% CI for seroresponse rate difference ≥70%.GMTa
VIMKUNYA
N=189
(95% CI)GMTa
Placebo
N=183
(95% CI)GMT Ratioa,e,
VIMKUNYA
over Placebo
(95% CI)721.0
(582.3, 892.7)8.08
(6.5, 10.0)89.2
(68.4, 116.4)Seroresponse
Rateb,d
VIMKUNYA
N=189
% (95% CI)Seroresponse
Rateb,d
Placebo
N=183
% (95% CI)Seroresponse Rate
Differencec,f
VIMKUNYA
minus Placebo
(95% CI)87.3
(81.8, 91.3)1.1
(0.3, 3.9)86.2
(80.0, 90.3)The seroresponse rate at 183 days post-vaccination in Study 1 was 85.5% among VIMKUNYA participants and 1.5% among placebo participants (seroresponse rate difference: 84.0% [95% CI: 81.7, 85.6]). The seroresponse rate at 183 days post-vaccination in Study 2 was 75.5% among VIMKUNYA participants and 1.2% among placebo participants (seroresponse rate difference: 74.4% [95% CI: 67.1, 80.1]).
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
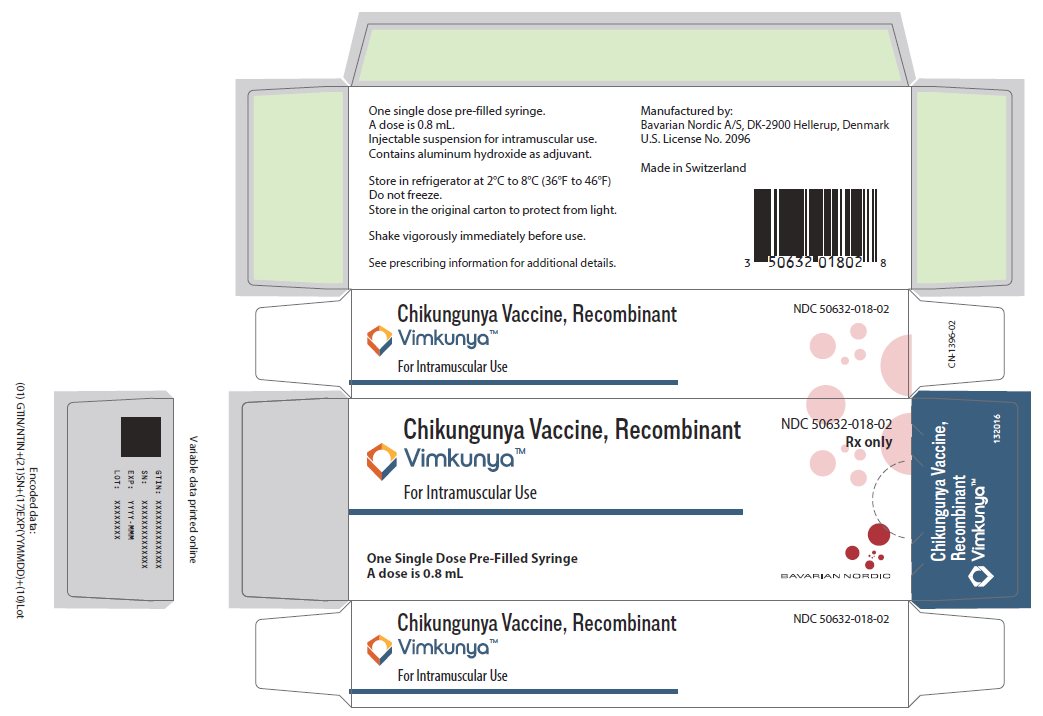
VIMKUNYA is supplied in a carton (NDC: 50632-018-02) containing one single dose pre-filled syringe (NDC: 50632-018-04) (packaged without needles).
Store VIMKUNYA in a refrigerator at 2°C to 8°C (36°F to 46°F). Store in the original carton to protect from light. DO NOT FREEZE.
VIMKUNYA may be held at room temperature (up to 25°C or 77°F) for up to 2 hours after removal from refrigerator. Discard the vaccine if not used within 2 hours after removal from refrigerator.
-
17 PATIENT COUNSELING INFORMATION
Inform the vaccine recipient:
- about the potential benefits and risks associated with vaccination with VIMKUNYA.
- that vaccination with VIMKUNYA may not protect all vaccine recipients and that personal precautions should be taken to reduce exposure to mosquito bites (e.g., adequate clothing, use of repellents, mosquito nets).
Instruct the vaccine recipient to report any adverse reactions to their health care provider, the vaccine manufacturer at 1-833-365-9596 (or online at drug.safety@bavarian-nordic.com), or through the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 (or online at www.vaers.hhs.gov).
Encourage women exposed to VIMKUNYA around the time of conception or during pregnancy to enroll in the pregnancy registry by calling 1-888-230-2491 or by visiting bnpregnancyregistry.com [See Use in Specific Populations (8.1)].
______________________________________________________________________________________________
Manufactured by:
Bavarian Nordic A/S
Philip Heymans Alle 3
2900 Hellerup, DenmarkU.S. License No. 2096
BAVARIAN NORDIC
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 50632-018-02
Rx onlyChikungunya Vaccine, Recombinant
VimkunyaTM
For Intramuscular UseOne Single Dose Pre-Filled Syringe
A dose is 0.8 mLBAVARIAN NORDIC
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VIMKUNYA
chikungunya vaccine, recombinant injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 50632-018 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 CAPSID PROTEIN (UNII: Y47FSH4J3N) (CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 CAPSID PROTEIN - UNII:Y47FSH4J3N) CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 CAPSID PROTEIN 10 ug in 0.8 mL CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E1 (UNII: VR2F8ZH764) (CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E1 - UNII:VR2F8ZH764) CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E1 15 ug in 0.8 mL CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E2 (UNII: YS6E2GD8XA) (CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E2 - UNII:YS6E2GD8XA) CHIKUNGUNYA VIRUS SENEGAL STRAIN 37997 ENVELOPE PROTEIN E2 15 ug in 0.8 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) SUCROSE (UNII: C151H8M554) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50632-018-02 1 in 1 CARTON 1 NDC: 50632-018-04 0.8 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125820 03/01/2025 Labeler - Bavarian Nordic A/S (310209754) Establishment Name Address ID/FEI Business Operations Bavarian Nordic Berna GmbH 480030654 MANUFACTURE(50632-018) , ANALYSIS(50632-018) Establishment Name Address ID/FEI Business Operations Grand River Aseptic Manufacturing Inc 005593490 MANUFACTURE(50632-018) , ANALYSIS(50632-018) Establishment Name Address ID/FEI Business Operations Grand River Aseptic Manufacturing Inc. 117925582 ANALYSIS(50632-018) Establishment Name Address ID/FEI Business Operations Sharp Packaging Services, LLC 143696495 LABEL(50632-018) , PACK(50632-018) , MANUFACTURE(50632-018)
Trademark Results [VIMKUNYA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIMKUNYA 97118110 not registered Live/Pending |
Emergent Travel Health Inc. 2021-11-10 |
 VIMKUNYA 79403374 not registered Live/Pending |
BAVARIAN NORDIC A/S 2024-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.