PERCORTEN V- desoxycorticosterone pivalatae injection, suspension
Percorten V by
Drug Labeling and Warnings
Percorten V by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc. , Sicor Societa' Italiana, Micro-Macinazione SA, AAIPharma Services Corp., AAIPharma Services Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- CAUTION:
-
DESCRIPTION:
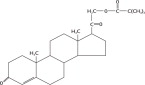
The active ingredient in PERCORTEN-V is desoxycorticosterone pivalate (DOCP). It is a mineralocorticoid hormone and an analog of desoxycorticosterone. It is white, odorless, and stable in air. It is practically insoluble in water, sparingly soluble in acetone, slightly soluble in methanol, ether and vegetable oils. The molecular weight is 414.58. It is designated chemically as 21 (2,2-dimethyl-1-oxopropoxy)-pregn-4-ene-3,20-dione. The empirical formula is C26H38O4 and the structural formula is:
PERCORTEN-V is a white aqueous suspension. Each ml contains 25mg desoxycorticosterone pivalate, 10.5mg methylcellulose, 3mg sodium carboxymethylcellulose, 1mg polysorbate 80 and 8mg sodium chloride with 0.002% thimerosal as preservative in water for injection.
-
CLINICAL PHARMACOLOGY:
Desoxycorticosterone pivalate (DOCP), like other adrenocorticoid hormones, is thought to act by controlling the rate of synthesis of proteins. It reacts with receptor proteins in the cytoplasm to form a steroid-receptor complex. This complex moves into the nucleus, where it binds to chromatin that results in genetic transcription of cellular DNA to messenger RNA. The steroid hormones appear to induce transcription and synthesis of specific proteins which produce the physiologic effects seen after administration.
DOCP is a long-acting ester of desoxycorticosterone acetate (DOCA) which is recognized as having the same qualitative effects as the natural mineralocorticoid hormone aldosterone.
The most important effect of DOCP is to increase the rate of renal tubular absorption of sodium. This effect is seen most intensely in the thick portion of the ascending limb of the loop of Henle. It also increases sodium absorption in the proximal convoluted tubule but this effect is less important in sodium retention. Chloride follows the sodium out of the renal tubule.
Another important effect of DOCP is enhanced renal excretion of potassium. This effect is driven by the resorption of sodium that pulls potassium from the extracellular fluid into the renal tubules, thus promoting potassium excretion.
DOCP also acts to increase extracellular fluid volume. The enhanced retention of sodium, chloride and bicarbonate, creates an osmotic gradient that promotes water absorption from the renal tubules. The extracellular fluid volume is supported. This expands the blood volume and improves the venous return to the heart and cardiac output. The expanded blood volume and increased cardiac output may result in elevated blood pressure. PERCORTEN-V prevents the life threatening hypotensive shock and pre-renal azotemia observed in animals suffering from hypoadrenocorticism.
The effects of PERCORTEN-V on electrolytes and extracellular fluid volume are dependent on a functioning kidney. Animals suffering from hypovolemia, pre-renal azotemia, and inadequate tissue perfusion must be rehydrated with intravenous fluid (saline) therapy, before starting PERCORTEN-V therapy. Primary renal disease should be ruled out before starting PERCORTEN-V therapy.
DOCP is an insoluble ester of desoxycorticosterone. The crystals are injected intramuscularly as a micro-crystalline depot where they slowly dissolve over time.
- INDICATION:
- WARNING:
-
PRECAUTIONS:
Some patients are more sensitive to the actions of PERCORTEN-V and may exhibit side effects in an exaggerated degree. Some patients may show signs of hypernatremia or hypokalemia. The dosage of PERCORTEN-V should be reduced in these patients. Concomitant use of PERCORTEN-V with potassium-sparing diuretics, such as spironolactone, may counter the effect of PERCORTEN-V because desoxycorticosterone pivalate and potassium-sparing diurectics exhibit opposing mechanisms of action.
Like other adrenocortical hormones, PERCORTEN-V may cause severe side effects if dosage is too high or prolonged. It may cause polyuria, polydipsia, increased blood volume, edema and cardiac enlargement. Excessive weight gain may indicate fluid retention secondary to sodium retention. PERCORTEN-V should be used with caution in patients with congestive heart disease, edema or renal disease.
-
ADVERSE REACTIONS (in controlled clinical field studies):
The following adverse reactions have been reported following the use of PERCORTEN-V: depression, polyuria, polydipsia, anorexia, skin and coat changes, diarrhea, vomiting, weakness, weight loss, incontinence, pain on injection and injection site abscess. Some of these effects may resolve with adjustments in dose or interval of PERCORTEN-V or concomitant glucocorticoid medication.
Post-Approval Experience, Rev. 2011:
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA-CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events are listed in decreasing order of reporting frequency in dogs: depression/lethargy, vomiting, anorexia, polydipsia, polyuria, diarrhea, facial/ muzzle edema, weakness, urticaria and anaphylaxis. Anemia has been reported following DOCP administration. For a complete listing of adverse reactions for desoxycorticosterone pivalate injectable suspension reported to CVM see:
http://www.fda.gov/AnimalVeterinary.
To report adverse effects, access medical information, or obtain additional product information call 1-888-545-5973. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae
-
EFFICACY:
PERCORTEN-V given intramuscularly at the appropriate dose and interval, is effective in replacing the mineralocorticoid deficit in dogs suffering from primary hypoadrenocorticism.
Results of two 75-day clinical studies in dogs with primary hypoadrenocorticism have demonstrated the clinical efficacy of PERCORTEN-V. Each dog received three doses of PERCORTEN-V (on days 0, 25 and 50). The results are summarized below.
Clinical Study Number 01 02 Number of Dogs 49 18 Average Diagnostic Values: Serum Sodium (mEq/L) 128.4 130.72 Serum Potassium (mEq/L) 7.28 7.47 Sodium/Potassium Ratio 18.09 17.86 ACTH Stimulation Test: Cortisol Resting (μg/dI) 0.28 0.68 Cortisol Post Stimulation (μg/dI) 0.27 1.34 Average PERCORTEN-V Dose (mg/lb): Day 0 0.97 0.99 Day 25 0.96 0.99 Day 50 0.94 0.97 Concomitant Glucocorticoid (Pred) 47% 39% Sodium/Potassium Ratios Day 0 25.18 26.42 Day 14 36.36 – Day 25 29.64 – Day 39 34.94 – Day 50 30.33 – Day 64 35.30 – Day 75 30.32 30.59 % Efficacy Therapy 96% 100% Case Management:1,2
An accurate diagnosis of primary canine adrenocortical insufficiency is of paramount importance for treatment success and should be established before initiation of PERCORTEN-V therapy. While hyponatremia and hyperkalemia are highly suggestive of adrenocortical insufficiency, they are not pathognomonic. A definitive diagnosis can only be made with an ACTH stimulation test. At diagnosis, classic cases of canine adrenocortical insufficiency may include clinical signs. Those signs are anorexia, lethargy, depression, weakness, vomiting and/or regurgitation, weight loss, diarrhea and collapse, serum sodium values less than 135 mEq/L, serum potassium greater than 6 mEq/L, sodium/potassium ratios below 25:1, plasma or serum cortisol concentration less than 4 μg/dl pre-and-post ACTH administration. Once the diagnosis is made, immediate therapy must be given to normalize electrolyte imbalance, correct hypovolemic shock and re-establish normal homeostasis. Such therapy should include large volumes of intravenous physiologic saline, glucocorticoids (i.e., prednisolone, dexamethasone) at shock doses and PERCORTEN-V. Anemia (normocytic, normochromic) may be present at diagnosis, but not identified due to hemoconcentration. Rapid rehydration may reduce the circulating red blood cell count to life-threatening levels in animals with preexisting anemia. Once the acute crisis has passed, renal and cardiovascular function should return to normal. Patient monitoring and dose adjustments of PERCORTEN-V and glucocorticoids should be instituted at this time (see Dosage).
-
SAFETY:3
In a laboratory study the safety of PERCORTEN-V was established in five month old Beagle dogs. PERCORTEN-V was administered IM to 24 Beagles at 0, 2.2, 6.6 or 11 mg/kg of body weight daily over a consecutive 3-day period every 28 days (equivalent to a cumulative monthly dosage of 0, 6.6, 19.8 or 33 mg/kg) for 6 months. This resulted in no mortality or any significant effects on body weight, food consumption, and ophthalmic observations at any dose level. However, polyuria and polydipsia were noted and creatinine concentration decreased (14-89 mg/dl) in the 1X, 3X and 5X groups. Histopathological changes were only observed in the kidneys when PERCORTEN-V was administered at ≥ 6.6 mg/kg. The primary renal lesion consisted of glomerulonephropathy seen in all males at ≥ 6.6 mg/kg, in one female at 6.6 mg/kg, and in all females at 11 mg/kg. Other possible treatment related lesions in the kidney, observed sporadically in the 6.6 and 11.0 mg/kg groups, were tubular hyperplasia, inflammation and tubular dilatation. Glomerulonephropathy may possibly be attributed to the pharmacological effects of the drug although there were no clinical measurements assessed in this study. In conclusion, PERCORTEN-V was well tolerated, when administered at 2.2 mg/kg on three consecutive days in every 28-day period for six months.
-
DOSAGE:1,2
In treating canine hypoadrenocorticism, PERCORTEN-V replaces the mineralocorticoid hormones only. Glucocorticoid replacement must be supplied by small daily doses of glucocorticoid hormones (e.g., prednisone or prednisolone) (0.2 – 0.4 mg/kg/day).
Dosage requirements are variable and must be individualized on the basis of the response of the patient to therapy. Begin treatment with PERCORTEN-V at a dose of 1.0 mg per pound of body weight every 25 days. In some patients the dose may be reduced. Serum sodium and potassium levels should be monitored to assure the animal is properly compensated. Most patients are well controlled with a dose range of 0.75 to 1.0 mg per pound of body weight, given every 21 to 30 days.
The well-controlled patient will have normal electrolytes at 14 days after administration or may exhibit slight hyponatremia and hyperkalemia. This needs no additional therapy as long as the patient is active and eating normally. Watch closely for depression, lethargy, vomiting or diarrhea which indicate a probable glucocorticoid deficiency.
At the end of the 25-day dosing interval, the patient should be clinically normal and have normal serum electrolytes. Alternatively, they may have slight hyponatremia and slight hyperkalemia. This constellation of signs indicate that the dosage and dosage interval should not be altered.
If the dog is not clinically normal or serum electrolytes are abnormal, then the dosage interval should be decreased 2-3 days.
Occasionally, dogs on PERCORTEN-V therapy may develop polyuria and polydipsia (PU/PD). This usually indicates excess glucocorticoid, but may also indicate a PERCORTEN-V excess. It is prudent to begin by decreasing the glucocorticoid dose first. If the PU/PD persists, then decrease the dose of PERCORTEN-V without changing the interval between doses.
Please note: Failure to administer glucocorticoids is the most common reason for treatment failure. Signs of glucocorticoid deficiency include depression, lethargy, vomiting and diarrhea. Such signs should be treated with high doses of injectable glucocorticoids (prednisolone or dexamethasone), followed by continued oral therapy 0.2 – 0.4 mg/kg/day. Oral supplementation with salt (NaCI) is not necessary with animals receiving PERCORTEN-V.
Guide to Maintenance Therapy
Starting Dose:
- DOCP 1 mg/lb every 25 days
- Prednisone 0.2 - 0.4 mg/kg/day
Guides for Adjustment:
- Clinical Problem/Solution
-
Polyuria/Polydipsia
- → decrease prednisone dose first,
- → then decrease DOCP dose,
- → do not change DOCP interval
- Depression, lethargy, vomiting or diarrhea
- → increase prednisone dose
- Hyperkalemia, Hyponatremia
- → decrease DOCP interval 2-3 days
-
ADMINISTRATION:
Before injection, shake the vial thoroughly to mix the microcrystals with the suspension vehicle. PERCORTEN-V suspension is to be injected intramuscularly. Care should be used to prevent inadvertent intravenous injection, which may cause acute collapse and shock. Such animals should receive immediate therapy for shock with intravenous fluids and glucocorticoids.
Once vial is broached, product should be used within 4 months.
- HOW SUPPLIED:
-
STORAGE:
Store at controlled room temperature 25ºC with excursions between 15-30ºC (59-86ºF) permitted. Protect from light. Protect from freezing.
References:
-
Canine and Feline Endocrinology and Reproduction. Second Edition (1996), E. C. Feldman and R. W. Nelson, W. B.
Saunders Co., New York and Third Edition (2004) Saunders/Elsevier, USA. -
Textbook of Veterinary Internal Medicine.
Fourth Edition, S. J. Ettinger and E. C. Feldman editors, W. B. Saunders Co., New York, 1995. - Toxicity of desoxycorticosterone pivalate given at high doses to clinically normal Beagles for six months, E. Chow, W. R. Campbell, J. C. Turnier, R.C. Lynn and K. L. Pavkov, Am. J. Vet. Res. 54(11):1954-1961, 1993.
Manufactured for: Elanco US Inc.
Greenfield, IN 46140, USA
Approved by FDA under NADA # 141-029PC3810I
Percorten, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
PA102318X
-
Canine and Feline Endocrinology and Reproduction. Second Edition (1996), E. C. Feldman and R. W. Nelson, W. B.
- Principal Display Panel - 25 mg/1 mL Carton Label
-
PRINCIPAL DISPLAY PANEL - 25 MG/1 ML VIAL LABEL
Percorten™-V 25 mg/ml
(desoxycorticosterone pivalate injectable suspension)
For intramuscular administration only. Usual dosage: see package
insert. Thimerosal 0.002% added preservative in water for injection.Shake well before using. Store at room temperature.
Caution: Federal law restricts this drug to use by or on order
of a licensed veterinarian.Manufactured for: Elanco US Inc., Greenfield, IN 46140, USA
Approved by FDA under NADA # 141-029
PC3811B
YL102318X
Elanco
-
INGREDIENTS AND APPEARANCE
PERCORTEN V
desoxycorticosterone pivalatae injection, suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-5667 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESOXYCORTICOSTERONE PIVALATE (UNII: 16665T4A2X) (DESOXYCORTONE - UNII:40GP35YQ49) DESOXYCORTICOSTERONE PIVALATE 25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-5667-0 1 in 1 CARTON 1 4 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141029 01/12/1998 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations Sicor Societa' Italiana 429369713 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Micro-Macinazione SA 480918515 API MANUFACTURE Establishment Name Address ID/FEI Business Operations AAIPharma Services Corp. 832394733 MANUFACTURE Establishment Name Address ID/FEI Business Operations AAIPharma Services Corp 079646861 PACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.