PARAGARD T 380A- copper intrauterine device
ParaGard T 380A by
Drug Labeling and Warnings
ParaGard T 380A by is a Prescription medication manufactured, distributed, or labeled by CooperSurgical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
ParaGard® T 380A
INTRAUTERINE COPPER CONTRACEPTIVEP/N 5128520401
Rev. 11/2017
Rx only
PRESCRIBING INFORMATIONParaGard® T 380A Intrauterine Copper Contraceptive
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
ParaGard® T 380A Intrauterine Copper Contraceptive should be placed and removed only by healthcare professionals who are experienced with these procedures.
-
DESCRIPTION
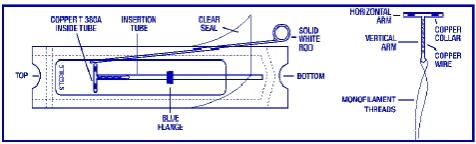
ParaGard® T 380A Intrauterine Copper Contraceptive (ParaGard®) is a T-shaped intrauterine device (IUD), measuring 32 mm horizontally and 36 mm vertically, with a 3 mm diameter bulb at the tip of the vertical stem. A monofilament polyethylene thread is tied through the tip, resulting in two white threads, each at least 10.5 cm in length, to aid in detection and removal of the device. The T-frame is made of polyethylene with barium sulfate to aid in detecting the device under x-ray. ParaGard® also contains copper: approximately 176 mg of wire coiled along the vertical stem and a 68.7 mg collar on each side of the horizontal arm. The total exposed copper surface area is 380 ± 23 mm2. One ParaGard® weighs less than one (1) gram. No component of ParaGard® or its packaging contains latex.
ParaGard® is packaged together with an insertion tube and solid white rod in a Tyvek® polyethylene pouch that is then sterilized. A moveable flange on the insertion tube aids in gauging the depth of insertion through the cervical canal and into the uterine cavity.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
ParaGard® is indicated for intrauterine contraception for up to 10 years. The pregnancy rate in clinical studies has been less than 1 pregnancy per 100 women each year.
Table 1: Percentage of women experiencing an unintended pregnancy during the first year of typical use and first year of perfect use of contraception and the percentage continuing use at the end of the first year: United States
% of Women Experiencing
an Accidental Pregnancy within
the First Year of Use
% of Women Continuing
Use at One Year3
Method (1)
Typical Use1 (2)
Perfect Use2 (3)
(4)
Chance4
85
85
Spermicides5
26
6
40
Periodic Abstinence
Calendar
Ovulation Method
Sympto-thermal6
Post-ovulation
25
9
3
2
1
63
Cap7
Parous women
Nulliparous women
40
20
26
9
42
56
Sponge
Parous women
Nulliparous women
40
20
20
9
42
56
Diaphragm7
20
6
56
Withdrawal
19
4
Condom8
Female (Reality)
Male
21
14
5
3
56
61
Pill
Progestin only
Combined
5
0.5
0.1
71
IUD
Progesterone T
Copper T 380A
LNg 20
2.0
0.8
0.1
1.5
0.6
0.1
81
78
81
Depo Provera
0.3
0.3
70
Norplant and Norplant-2
0.05
0.05
88
Female sterilization
0.5
0.5
100
Male sterilization
0.15
0.10
100
Emergency Contraceptive Pills: Treatment initiated within 72 hours after unprotected intercourse reduces the risk of pregnancy by at least 75%.9
Lactational Amenorrhea Method: LAM is a highly effective temporary method of contraception.10Footnotes to Table 1
Source: Trussel J, Contraceptive efficacy. In Hatcher RA, Trussel J, Stewart F, Cates W, Stewart GK, Kowal D, Guest F, Contraceptive Technology: Seventeenth Revised Edition. New York NY: Irvington Publishers, 1998.
- Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
- Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any reason.
- Among couples attempting to avoid pregnancy, the percentage who continue to use a method for one year.
- The percents becoming pregnant in columns (2) and (3) are based on data from populations where contraception is not used and from women who cease using contraception in order to become pregnant. Among such populations, about 89% become pregnant within one year. This estimate was lowered slightly (to 85%) to represent the percentage who would become pregnant within one year among women now relying on reversible methods of contraception if they abandoned contraception altogether.
- Foams, creams, gels, vaginal suppositories, and vaginal film.
- Cervical mucus (ovulation) method supplemented by calendar in the pre-ovulatory and basal body temperature in the post-ovulatory phases.
- With spermicidal cream or jelly.
- Without spermicides.
- The treatment schedule is one dose within 72 hours after unprotected intercourse, and a second dose 12 hours after the first dose. Preven is the only dedicated product specifically marketed for emergency contraception. The Food and Drug Administration has also declared the following brands of oral contraceptive to be safe and effective for emergency contraception: Ovral (1 dose is 2 white pills), Alesse (1 dose is 5 pink pills), Nordette or Levlen (1 dose is 4 light-orange pills), Lo/Ovral (1 dose is 4 white pills), Triphasil or Tri-Levlen (1 dose is 4 yellow pills).
- However, to maintain effective protection against pregnancy, another method of contraception must be used as soon as menstruation resumes, the frequency or duration of breastfeeds is reduced, bottle feeds are introduced, or the baby reaches 6 months of age.
- Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason.
-
CONTRAINDICATIONS
ParaGard® should not be placed when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy
- Abnormalities of the uterus resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease, or current behavior suggesting a high risk for pelvic inflammatory disease
- Postpartum endometritis or postabortal endometritis in the past 3 months
- Known or suspected uterine or cervical malignancy
- Genital bleeding of unknown etiology
- Mucopurulent cervicitis
- Wilson’s disease
- Allergy to any component of ParaGard®
- A previously placed IUD that has not been removed
- Pregnancy or suspicion of pregnancy
-
WARNINGS
1. Intrauterine Pregnancy
If intrauterine pregnancy occurs with ParaGard® in place and the string is visible, ParaGard® should be removed because of the risk of spontaneous abortion, premature delivery, sepsis, septic shock, and, rarely, death. Removal may be followed by pregnancy loss.
If the string is not visible, and the woman decides to continue her pregnancy, check if the ParaGard® is in her uterus (for example, by ultrasound). If ParaGard® is in her uterus, warn her that there is an increased risk of spontaneous abortion and sepsis, septic shock, and rarely, death.1 In addition, the risk of premature labor and delivery is increased.1
Human data about risk of birth defects from copper exposure are limited. However, studies have not detected a pattern of abnormalities, and published reports do not suggest a risk that is higher than the baseline risk for birth defects.
2. Ectopic Pregnancy
Women who become pregnant while using ParaGard® should be evaluated for ectopic pregnancy. A pregnancy that occurs with ParaGard® in place is more likely to be ectopic than a pregnancy in the general population. However, because ParaGard® prevents most pregnancies, women who use ParaGard® have a lower risk of an ectopic pregnancy than sexually active women who do not use any contraception.2-3
3. Pelvic Infection
Although pelvic inflammatory disease (PID) in women using IUDs is uncommon, IUDs may be associated with an increased relative risk of PID compared to other forms of contraception and to no contraception. The highest incidence of PID occurs within 20 days following insertion. Therefore, the visit following the first post-insertion menstrual period is an opportunity to assess the patient for infection, as well as to check that the IUD is in place. (See INSTRUCTIONS FOR USE, Continuing Care.) Since pelvic infection is most frequently associated with sexually transmitted organisms, IUDs are not recommended for women at high risk for sexual infection. Prophylactic antibiotics at the time of insertion do not appear to lower the incidence of PID.4
PID can have serious consequences, such as tubal damage (leading to ectopic pregnancy or infertility), hysterectomy, sepsis, and, rarely, death. It is therefore important to promptly assess and treat any woman who develops signs or symptoms of PID.
Guidelines for treatment of PID are available from the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia at www.cdc.gov or 1-800-311-3435. Antibiotics are the mainstay of therapy. Most healthcare professionals also remove the IUD.
The significance of actinomyces-like organisms on Papanicolaou smear in an asymptomatic IUD user is unknown,5-6 and so this finding alone does not always require IUD removal and treatment. However, because pelvic actinomycosis is a serious infection, a woman who has symptoms of pelvic infection possibly due to actinomyces should be treated and have her IUD removed.
4. Immunocompromise
Women with AIDS should not have IUDs inserted unless they are clinically stable on antiretroviral therapy. Limited data suggest that asymptomatic women infected with human immunodeficiency virus may use intrauterine devices. Little is known about the use of IUDs in women who have illnesses causing serious immunocompromise. Therefore these women should be carefully monitored for infection if they choose to use an IUD. The risk of pregnancy should be weighed against the theoretical risk of infection.
5. Embedment
Partial penetration or embedment of ParaGard® in the myometrium can make removal difficult. In some cases, surgical removal may be necessary.
6. Perforation
Partial or total perforation of the uterine wall or cervix may occur rarely during placement, although it may not be detected until later. Spontaneous migration has also been reported. If perforation does occur, remove ParaGard® promptly, since the copper can lead to intraperitoneal adhesions. Intestinal penetration, intestinal obstruction, and/or damage to adjacent organs may result if an IUD is left in the peritoneal cavity. Pre-operative imaging followed by laparoscopy or laparotomy is often required to remove an IUD from the peritoneal cavity.
-
PRECAUTIONS
Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
1. Information for patients
Before inserting ParaGard® discuss the Patient Package Insert with the patient, and give her time to read the information. Discuss any questions she may have concerning ParaGard® as well as other methods of contraception. Instruct her to promptly report symptoms of infection, pregnancy, or missing strings.
3. Vaginal bleeding
In the 2 largest clinical trials with ParaGard® (see ADVERSE REACTIONS, Table 2), menstrual changes were the most common medical reason for discontinuation of ParaGard®. Discontinuation rates for pain and bleeding combined are highest in the first year of use and diminish thereafter. The percentage of women who discontinued ParaGard® because of bleeding problems or pain during these studies ranged from 11.9% in the first year to 2.2 % in year 9. Women complaining of heavy vaginal bleeding should be evaluated and treated, and may need to discontinue ParaGard®. (See ADVERSE REACTIONS.)
4. Vasovagal reactions, including fainting
Some women have vasovagal reactions immediately after insertion. Hence, patients should remain supine until feeling well and should be cautious when getting up.
5. Expulsion following placement after a birth or abortion
ParaGard® has been placed immediately after delivery, although risk of expulsion may be higher than when ParaGard® is placed at times unrelated to delivery.7 However, unless done immediately postpartum, insertion should be delayed to the second postpartum month because insertion during the first postpartum month (except for immediately after delivery) has been associated with increased risk of perforation.8
ParaGard® can be placed immediately after abortion, although immediate placement has a slightly higher risk of expulsion than placement at other times.9 Placement after second trimester abortion is associated with a higher risk of expulsion than placement after the first trimester abortion.9
6. Magnetic resonance imaging (MRI)
Limited data suggest that MRI at the level of 1.5 Tesla is acceptable in women using ParaGard®. One study examined the effect of MRI on the CU-7® Intrauterine Copper Contraceptive and Lippes Loop™ intrauterine devices. Neither device moved under the influence of the magnetic field or heated during the spin-echo sequences usually employed for pelvic imaging.10 An in vitro study did not detect movement or temperature change when ParaGard® was subjected to MRI.11
7. Medical diathermy
Theoretically, medical (non-surgical) diathermy (short-wave and microwave heat therapy) in a patient with a metal-containing IUD may cause heat injury to the surrounding tissue. However, a small study of eight women did not detect a significant elevation of intrauterine temperature when diathermy was performed in the presence of a copper IUD.12
9. Nursing mothers
Nursing mothers may use ParaGard®. No difference has been detected in concentration of copper in human milk before and after insertion of copper IUDs. The literature is conflicting, but limited data suggest that there may be an increased risk of perforation and expulsion if a woman is lactating. 13
-
ADVERSE REACTIONS
The most serious adverse events associated with intrauterine contraception are discussed in WARNINGS and PRECAUTIONS. These include:
Intrauterine pregnancy
Pelvic infection
Septic abortion
Perforation
Ectopic pregnancy
EmbedmentTable 2 shows discontinuation rates from two clinical studies by adverse event and year.
Table 2. Summary of Rates (No. per 100 Subjects) by Year for Adverse Events Causing Discontinuation
Adverse Event
Year
1
2
3
4
5
6
7
8
9
10
Pregnancy
0.7
0.3
0.6
0.2
0.3
0.2
0.0
0.4
0.0
0.0
Expulsion
5.7
2.5
1.6
1.2
0.3
0.0
0.6
1.7
0.2
0.4
Bleeding/Pain
11.9
9.8
7.0
3.5
3.7
2.7
3.0
2.5
2.2
3.7
Other Medical Event
2.5
2.1
1.6
1.7
0.1
0.3
1.0
0.4
0.7
0.3
No. of Women at Start of Year
4932
3149
2018
1121
872
621
563
483
423
325*Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the Population Council (3,536 subjects) and the World Health Organization (1,396 subjects) trials.
The following adverse events have also been observed. These are listed alphabetically and not by order of frequency or severity.
Table 2. Summary of Rates (No. per 100 Subjects) by Year for Adverse Events Causing Discontinuation
Anemia
Menstrual flow, prolonged
Backache
Menstrual spotting
Dysmenorrhea
Pain and cramping
Dyspareunia
Urticarial allergic skin reaction
Expulsion, complete or partial
Vaginitis
Leukorrhea -
INSTRUCTIONS FOR USE
The placement technique for ParaGard® is different from that used for other IUDs. Therefore, the clinician should be familiar with the following instructions.
ParaGard® may be placed at any time during the cycle when the clinician is reasonably certain the patient is not pregnant. For information about timing of postpartum and postabortion insertions, see PRECAUTIONS.
A single ParaGard® should be placed at the fundus of the uterine cavity. ParaGard® should be removed on or before 10 years from the date of insertion.
Before Placement:
- Make sure that the patient is an appropriate candidate for ParaGard® and that she has read the Patient Package Insert.
- Use of an analgesic before insertion is at the discretion of the patient and the clinician.
- Establish the size and position of the uterus by pelvic examination.
- Insert a speculum and cleanse the vagina and cervix with an antiseptic solution.
- Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
- Gently insert a sterile sound to measure the depth of the uterine cavity.
- The uterus should sound to a depth of 6 to 9 cm except when inserting ParaGard ® immediately post-abortion or post-partum. Insertion of ParaGard® into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation. If you encounter cervical stenosis, avoid undue force. Dilators may be helpful in this situation.
How to Load and Place ParaGard®:
Do not bend the arms of ParaGard® earlier than 5 minutes before it is to be placed in the uterus. Use aseptic technique when handling ParaGard® and the part of the insertion tube that will enter the uterus.
STEP 1
Load ParaGard® into the insertion tube by folding the two horizontal arms of ParaGard® against the stem and push the tips of the arms securely into the inserter tube.
If you do not have sterile gloves, you can do STEPS 1 and 2 while ParaGard® is in the sterile package. First, place the package face up on a clean surface. Next, open at the bottom end (where arrow says OPEN). Pull the solid white rod partially from the package so it will not interfere with assembly. Place thumb and index finger on top of package on ends of the horizontal arms. Use other hand to push insertion tube against arms of ParaGard® (shown by arrow in Fig. 1). This will start bending the T arms.
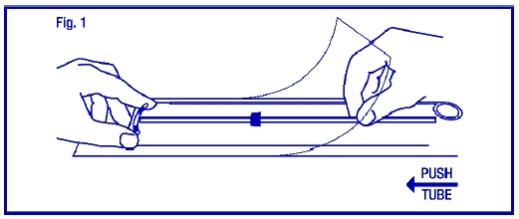
STEP 2
Bring the thumb and index finger closer together to continue bending the arms until they are alongside the stem. Use the other hand to withdraw the insertion tube just enough so that the insertion tube can be pushed and rotated onto the tips of the arms. Your goal is to secure the tips of the arms inside the tube (Fig. 2). Insert the arms no further than necessary to insure retention. Introduce the solid white rod into the insertion tube from the bottom, alongside the threads, until it touches the bottom of the ParaGard®.
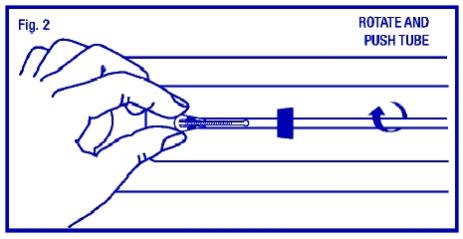
STEP 3
Grasp the insertion tube at the open end of the package; adjust the blue flange so that the distance from the top of the ParaGard® (where it protrudes from the inserter) to the blue flange is the same as the uterine depth that you measured with the sound. Rotate the insertion tube so that the horizontal arms of the T and the long axis of the blue flange lie in the same horizontal plane (Fig. 3). Now pass the loaded insertion tube through the cervical canal until ParaGard® just touches the fundus of the uterus. The blue flange should be at the cervix in the horizontal plane.
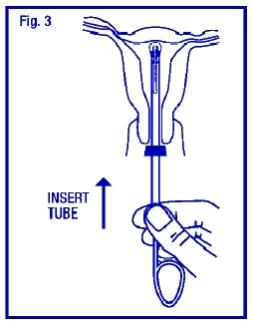
STEP 4
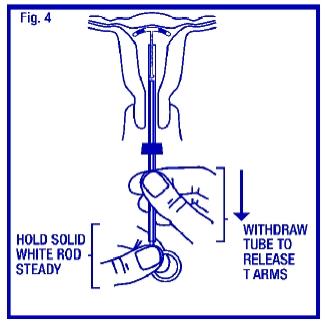
To release the arms of ParaGard®, hold the solid white rod steady and withdraw the insertion tube no more than one centimeter. This releases the arms of ParaGard® high in the uterine fundus (Fig. 4).
STEP 5
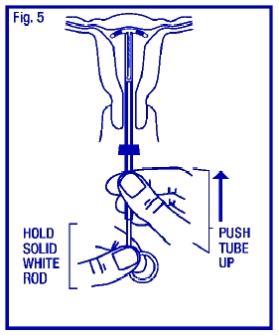
Gently and carefully move the insertion tube upward toward the top of the uterus, until slight resistance is felt. This will ensure placement of the T at the highest possible position within the uterus (Fig. 5).
STEP 6
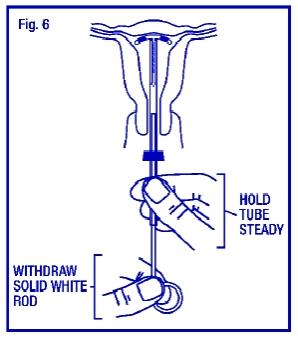
Hold the insertion tube steady and withdraw the solid white rod (Fig. 6).
STEP 7
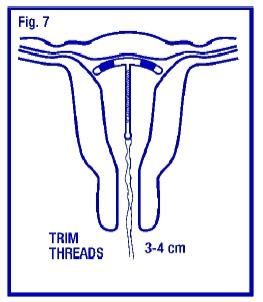
Gently and slowly withdraw the insertion tube from the cervical canal. Only the threads should be visible protruding from the cervix. (Fig. 7). Trim the threads so that 3 to 4 cm protrude into the vagina. Note the length of the threads in the patient’s records.
If you suspect that ParaGard® is not in the correct position, check placement (with ultrasound, if necessary). If ParaGard® is not positioned completely within the uterus, remove it and replace it with a new ParaGard®. Do not reinsert an expelled or partially expelled ParaGard®.
- Make sure that the patient is an appropriate candidate for ParaGard® and that she has read the Patient Package Insert.
-
CAUTION
Instrumentation of the cervical os may result in vasovagal reactions, including fainting. Have the patient remain supine until she feels well, and have her get up with caution.
Continuing Care:
Following placement, examine the patient after her first menses to confirm that ParaGard® is still in place. You should be able to see or feel only the threads. If ParaGard® has been partially or completely expelled, remove it. You can place a new ParaGard® if the patient desires and if she is not pregnant. Do not reinsert a used ParaGard®.
Evaluate the patient promptly if she complains of any of the following:
- Abdominal or pelvic pain, cramping, or tenderness; malodorous discharge; bleeding; fever
- A missed period
(See WARNINGS, Pelvic Infection, Intrauterine Pregnancy and Ectopic Pregnancy.)
The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, or pregnancy.
If you cannot find the threads in the vagina, check that ParaGard® is still in the uterus. The threads can retract into the uterus or break, or ParaGard® can break, perforate the uterus, or be expelled. Gentle probing of the cavity, radiography, or sonography may be required to locate the IUD.
If there is evidence of partial expulsion, perforation, or breakage, remove ParaGard®.
How to Remove ParaGard®
Remove ParaGard® with forceps, pulling gently on the exposed threads. The arms of ParaGard® will fold upwards as it is withdrawn from the uterus. You may immediately insert a new ParaGard® if the patient requests it and has no contraindications.
Embedment or breakage of ParaGard® in the myometrium can make removal difficult. Analgesia, paracervical anesthesia, and cervical dilation may assist in removing an embedded ParaGard®. An alligator forceps or other grasping instrument may be helpful. Hysteroscopy may also be helpful.
- Abdominal or pelvic pain, cramping, or tenderness; malodorous discharge; bleeding; fever
-
HOW SUPPLIED
ParaGard® is available in cartons of 1 (one) sterile unit (NDC: 59365-5128-1). Each ParaGard® is packaged together with an insertion tube and solid white rod in a Tyvek® polyethylene pouch.
-
REFERENCES
- Tatum HJ, Schmidt FH, Jain AK. Management and outcome of pregnancies associated with the Copper T intrauterine contraceptive device. Am J Obstet Gynecol. 1976;126:869-879.
- Sivin I. Dose- and age-dependent ectopic pregnancy risks with intrauterine contraception. Obstet Gynecol. 1991;78:291-298.
- Franks AL, Beral V, Cates W Jr, Hogue CJR. Contraception and ectopic pregnancy risk. Am J Obstet Gynecol. 1990;163:1120-1123.
- Grimes DA, Schulz KF. Prophylactic antibiotics for intrauterine device insertion: a meta-analysis of the randomized controlled trials. Contraception. 1999;60:57-63.
- Lippes J. Pelvic actinomycosis: a review and preliminary look at prevalence. Am J Obstet Gynecol. 1999;180:265-269.
- Petitti DB, Yamamoto D, Morgenstern N. Factors associated with actinomyces-like organisms on Papanicolaou smear in users of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983;145:338-341.
- Grimes D, Schulz K, van Vliet H, Stanwood N. Immediate post-partum insertion of intrauterine devices: a Cochrane review. Hum Reprod. 2002;17:549-554.
- Cole LP, Edelman DA, Potts DM, Wheeler RG, Laufe LE. Postpartum insertion of modified intrauterine devices. J Reprod Med. 1984;29:677-682.
- Grimes DA, Schulz KF, Stanwood N. Immediate post-abortal insertion of intrauterine devices. (Cochrane Review). In: The Cochrane Library, Issue 2, 2003. Oxford: Update Software.
- Hess T, Stepanow B, Knopp MV. Magnetic resonance imaging: safety of intrauterine contraceptive devices during MR imaging. Eur Radiol. 1996;6:66-68.
- Mark AS, Hricak H. Intrauterine devices. MR imaging. Radiology. 1987;162:311-314.
- Heick A., Espersen T., Pedersen HL, Raahauge J: Is diathermy safe in women with copper-bearing IUDs? Acta Obstet Gynecol Scand. 1991;70(2):153-5.
- Rodrigues da Cunha AC, Dorea JG, Cantuaria AA. Intrauterine device and maternal copper metabolism during lactation. Contraception 2001;63:37-9.
- Tatum HJ, Schmidt FH, Jain AK. Management and outcome of pregnancies associated with the Copper T intrauterine contraceptive device. Am J Obstet Gynecol. 1976;126:869-879.
-
INFORMATION FOR PATIENTS
ParaGard® T 380A
Intrauterine Copper ContraceptiveParaGard® T 380A Intrauterine Copper Contraceptive is used to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
It is important for you to understand this brochure and discuss it with your healthcare provider before choosing ParaGard® T 380A Intrauterine Copper Contraceptive (ParaGard®). You should also learn about other birth control methods that may be an option for you.
What is ParaGard®?
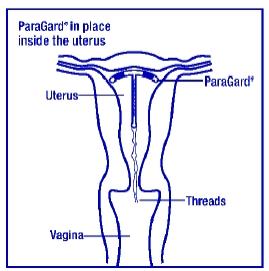
ParaGard® is a copper-releasing device that is placed in your uterus to prevent pregnancy for up to 10 years.
ParaGard® is made of white plastic in the shape of a “T.” Copper is wrapped around the stem and arms of the “T”. Two white threads are attached to the stem of the “T”. The threads are the only part of ParaGard® that you can feel when ParaGard® is in your uterus. ParaGard® and its components do not contain latex.

How long can I keep ParaGard ® in place?
You can keep ParaGard® in your uterus for up to 10 years. After 10 years, you should have ParaGard® removed by your healthcare provider. If you wish and if it is still right for you, you may get a new ParaGard® during the same visit.
What if I change my mind and want to become pregnant?
Your healthcare provider can remove ParaGard® at any time. After discontinuation of
ParaGard®, its contraceptive effect is reversed.How does ParaGard® work?
Ideas about how ParaGard® works include preventing sperm from reaching the egg, preventing sperm from fertilizing the egg, and possibly preventing the egg from attaching (implanting) in the uterus. ParaGard® does not stop your ovaries from making an egg (ovulating) each month.
How well does ParaGard® work?
Fewer than 1 in 100 women become pregnant each year while using ParaGard®.
The table below shows the chance of getting pregnant using different types of birth control. The numbers show typical use, which includes people who don't always use birth control correctly.
Number of women out of 100 women who are likely to get pregnant over one year
Method of birth control
Pregnancies per 100
women over one year
No Method
85
Spermicides
26
Periodic abstinence
25
Cap with Spermicides
20
Vaginal Sponge
20 to 40
Diaphragm with Spermicides
20
Withdrawal
19
Condom without spermicides (female)
21
Condom without spermicides (male)
14
Oral Contraceptives
5
IUDs, Depo-Provera,implants, sterilization
less than 1Who might use ParaGard®?
You might choose ParaGard® if you
- need birth control that is very effective
- need birth control that stops working when you stop using it
- need birth control that is easy to use
Who should not use ParaGard®?
You should not use ParaGard® if you
- Might be pregnant
- Have a uterus that is abnormally shaped inside
- Have a pelvic infection called pelvic inflammatory disease (PID) or have current behavior that puts you at high risk of PID (for example, because you are having sex with several men, or your partner is having sex with other women)
- Have had an infection in your uterus after a pregnancy or abortion in the past 3 months
- Have cancer of the uterus or cervix
- Have unexplained bleeding from your vagina
- Have an infection in your cervix
- Have Wilson’s disease (a disorder in how the body handles copper)
- Are allergic to anything in ParaGard®
- Already have an intrauterine contraceptive in your uterus
How is ParaGard® placed in the uterus?
ParaGard® is placed in your uterus during an office visit. Your healthcare provider first examines you to find the position of your uterus. Next, he or she will cleanse your vagina and cervix, measure your uterus, and then slide a plastic tube containing ParaGard® into your uterus. The tube is removed, leaving ParaGard® inside your uterus. Two white threads extend into your vagina. The threads are trimmed so they are just long enough for you to feel with your fingers when doing a self-check. As ParaGard® goes in, you may feel cramping or pinching. Some women feel faint, nauseated, or dizzy for a few minutes afterwards. Your healthcare provider may ask you to lie down for a while and to get up slowly.
How do I check that ParaGard® is in my uterus?
Visit your healthcare provider for a check-up about one month after placement to make sure ParaGard® is still in your uterus.
You can also check to make sure that ParaGard® is still in your uterus by reaching up to the top of your vagina with clean fingers to feel the two threads. Do not pull on the threads.
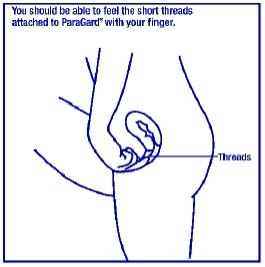
If you cannot feel the threads, ask your healthcare provider to check if ParaGard® is in the right place. If you can feel more of ParaGard® than just the threads, ParaGard® is not in the right place. If you can’t see your healthcare provider right away, use an additional birth control method. If ParaGard® is in the wrong place, your chances of getting pregnant are increased. It is a good habit for you to check that ParaGard® is in place once a month.
You may use tampons when you are using ParaGard®.
What if I become pregnant while using ParaGard®?
If you think you are pregnant, contact your healthcare professional right away. If you are pregnant and ParaGard® is in your uterus, you may get a severe infection or shock, have a miscarriage or premature labor and delivery, or even die. Because of these risks, your healthcare provider will recommend that you have ParaGard® removed, even though removal may cause miscarriage.
If you continue a pregnancy with ParaGard® in place, see your healthcare provider regularly. Contact your healthcare provider right away if you get fever, chills, cramping, pain, bleeding, flu-like symptoms, or an unusual, bad smelling vaginal discharge.
A pregnancy with ParaGard® in place has a greater than usual chance of being ectopic (outside your uterus). Ectopic pregnancy is an emergency that may require surgery. An ectopic pregnancy can cause internal bleeding, infertility, and death. Unusual vaginal bleeding or abdominal pain may be signs of an ectopic pregnancy.
Copper in ParaGard® does not seem to cause birth defects.
What side effects can I expect with ParaGard®?
The most common side effects of ParaGard® are heavier, longer periods and spotting between periods; most of these side effects diminish after 2-3 months. However, if your menstrual flow continues to be heavy or long, or spotting continues, contact your healthcare provider.
Infrequently, serious side effects may occur:
- Pelvic inflammatory disease (PID): Uncommonly, ParaGard® and other IUDs are associated with PID. PID is an infection of the uterus, tubes, and nearby organs. PID is most likely to occur in the first 20 days after placement. You have a higher chance of getting PID if you or your partner have sex with more than one person. PID is treated with antibiotics. However, PID can cause serious problems such as infertility, ectopic pregnancy, and chronic pelvic pain. Rarely, PID may even cause death. More serious cases of PID require surgery or a hysterectomy (removal of the uterus). Contact your healthcare provider right away if you have any of the signs of PID: abdominal or pelvic pain, painful sex, unusual or bad smelling vaginal discharge, chills, heavy bleeding, or fever.
- Difficult removals: Occasionally ParaGard® may be hard to remove because it is stuck in the uterus. Surgery may sometimes be needed to remove ParaGard®.
- Perforation: Rarely, ParaGard® goes through the wall of the uterus, especially during placement. This is called perforation. If ParaGard® perforates the uterus, it should be removed. Surgery may be needed. Perforation can cause infection, scarring, or damage to other organs. If ParaGard® perforates the uterus, you are not protected from pregnancy.
- Expulsion: ParaGard® may partially or completely fall out of the uterus. This is called expulsion. Women who have never been pregnant may be more likely to expel ParaGard® than women who have been pregnant before. If you think that ParaGard® has partly or completely fallen out, use an additional birth control method, such as a condom and call your healthcare provider.
You may have other side effects with ParaGard®. For example, you may have anemia (low blood count), backache, pain during sex, menstrual cramps, allergic reaction, vaginal infection, vaginal discharge, faintness, or pain. This is not a complete list of possible side effects. If you have questions about a side effect, check with your healthcare provider.
When should I call my healthcare provider?
Call your healthcare provider if you have any concerns about ParaGard®. Be sure to call if you
- Think you are pregnant
- Have pelvic pain or pain during sex
- Have unusual vaginal discharge or genital sores
- Have unexplained fever
- Might be exposed to sexually transmitted diseases (STDs)
- Cannot feel ParaGard®’s threads or can feel the threads are much longer
- Can feel any other part of the ParaGard® besides the threads
- Become HIV positive or your partner becomes HIV positive
- Have severe or prolonged vaginal bleeding
- Miss a menstrual period
General advice about prescription medicines
This brochure summarizes the most important information about ParaGard®. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider for information about ParaGard® that is written for healthcare professionals.
Checklist
This checklist will help you and your healthcare provider discuss the pros and cons of ParaGard® for you. Do you have any of the following conditions?
Yes
No
Don't know
Abnormal Pap smear
□
□
□
Abnormalities of the uterus
□
□
□
Allergy to copper
□
□
□
Anemia or blood clotting problems
□
□
□
Bleeding between periods
□
□
□
Cancer of the uterus or cervix
□
□
□
Fainting attacks
□
□
□
Genital sores
□
□
□
Heavy menstrual flow
□
□
□
HIV or AIDS
□
□
□
Infection of the uterus or cervix
□
□
□
IUD in place now or in the past
□
□
□
More than one sexual partner
□
□
□
Pelvic infection (PID)
□
□
□
Possible pregnancy
□
□
□
Repeated episodes of pelvic infection (PID)
□
□
□
Serious infection following a pregnancy or abortion in the past 3 months
□
□
□
Severe menstrual cramps
□
□
□
Sexual partner who has more than one sexual partner
□
□
□
Sexually transmitted disease (STD) such as gonorrhea or chlamydia
□
□
□
Wilson's disease
□
□
□CooperSurgical, Inc.
95 Corporate Drive, Trumbull, CT 06611 USA
Package/Label Display Panel
- need birth control that is very effective
-
PRINCIPAL DISPLAY PANEL
PARAGARD
Intrauterine Copper Contraceptive
1 Unit
ParaGard® T380A Intrauterine Copper Contraceptive Single Carton Text
CooperSurgical, Inc.
95 Corporate Drive, Trumbull, CT 06611 USA
1
UNIT
PARAGARD ® T380A
intrauterine copper contraceptive
NDC 59365-5128-1
Each unit wound with approximately 176 mg of copper wire. In addition, a single copper sleeve is swaged on each of the two transverse arms. Each sleeve contains approximately 68.7 mg of copper.
The total surface area of copper on the device is 380 ± 23mm2. IMPORTANT: To be inserted in the uterus only by a licensed clinician or under the supervision of a physician. See detailed instructions
for use. NOTE: A Patient Package Insert is provided with each unit. Please ensure that this package insert is provided to the patient. Review patient brochure with each patient before insertion.
Store at controlled room temperature: 59° to 86°F (15° to 30°C)
CooperSurgical, Inc., 95 Corporate Drive, Trumbull, CT 06611
-
INGREDIENTS AND APPEARANCE
PARAGARD T 380A
copper intrauterine deviceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59365-5128 Route of Administration INTRAUTERINE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 313.4 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) BARIUM SULFATE (UNII: 25BB7EKE2E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59365-5128-1 1 in 1 CARTON 11/02/2017 1 1 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018680 11/02/2017 Labeler - CooperSurgical, Inc. (801895244)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.